Costimulation blockade in combination with IL-2 permits regulatory T cell sparing immunomodulation that inhibits autoimmunity
- PMID: 36347877
- PMCID: PMC9643453
- DOI: 10.1038/s41467-022-34477-1
Costimulation blockade in combination with IL-2 permits regulatory T cell sparing immunomodulation that inhibits autoimmunity
Erratum in
-
Author Correction: Costimulation blockade in combination with IL-2 permits regulatory T cell sparing immunomodulation that inhibits autoimmunity.Nat Commun. 2023 Sep 15;14(1):5724. doi: 10.1038/s41467-023-41483-4. Nat Commun. 2023. PMID: 37714833 Free PMC article. No abstract available.
Abstract
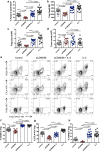
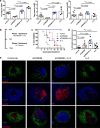
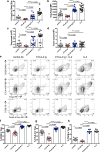
Blockade of CD28 costimulation with CTLA-4-Ig/Abatacept is used to dampen effector T cell responses in autoimmune and transplantation settings. However, a significant drawback of this approach is impaired regulatory T cell homeostasis that requires CD28 signaling. Therefore, strategies that restrict the effects of costimulation blockade to effector T cells would be advantageous. Here we probe the relative roles of CD28 and IL-2 in maintaining Treg. We find provision of IL-2 counteracts the regulatory T cell loss induced by costimulation blockade while minimally affecting the conventional T cell compartment. These data suggest that combining costimulation blockade with IL-2 treatment may selectively impair effector T cell responses while maintaining regulatory T cells. Using a mouse model of autoimmune diabetes, we show combined therapy supports regulatory T cell homeostasis and protects from disease. These findings are recapitulated in humanised mice using clinically relevant reagents and provide an exemplar for rational use of a second immunotherapy to offset known limitations of the first.
© 2022. The Author(s).
Conflict of interest statement
R.J.H., D.A.S. and A.Fr. declare interests in developing IL-2 therapeutics at Roche. R.J.H. and D.A.S. and A.Fr. are shareholders in Roche. The other authors declare no competing interests.
Figures



Similar articles
-
Challenges and opportunities in targeting the CD28/CTLA-4 pathway in transplantation and autoimmunity.Expert Opin Biol Ther. 2017 Aug;17(8):1001-1012. doi: 10.1080/14712598.2017.1333595. Epub 2017 May 30. Expert Opin Biol Ther. 2017. PMID: 28525959 Free PMC article. Review.
-
Costimulation blockade and Tregs in solid organ transplantation.Front Immunol. 2022 Sep 2;13:969633. doi: 10.3389/fimmu.2022.969633. eCollection 2022. Front Immunol. 2022. PMID: 36119115 Free PMC article. Review.
-
Selective CD28 blockade attenuates CTLA-4-dependent CD8+ memory T cell effector function and prolongs graft survival.JCI Insight. 2018 Jan 11;3(1):e96378. doi: 10.1172/jci.insight.96378. eCollection 2018 Jan 11. JCI Insight. 2018. PMID: 29321374 Free PMC article.
-
Selective blockade of CD28 on human T cells facilitates regulation of alloimmune responses.JCI Insight. 2017 Oct 5;2(19):e89381. doi: 10.1172/jci.insight.89381. JCI Insight. 2017. PMID: 28978798 Free PMC article.
-
Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells.PLoS One. 2013 Dec 23;8(12):e83139. doi: 10.1371/journal.pone.0083139. eCollection 2013. PLoS One. 2013. PMID: 24376655 Free PMC article.
Cited by
-
Desensitization and belatacept-based maintenance therapy in pregnancy-sensitized monkeys receiving a kidney transplant.Sci Adv. 2023 May 19;9(20):eadg1448. doi: 10.1126/sciadv.adg1448. Epub 2023 May 19. Sci Adv. 2023. PMID: 37205758 Free PMC article.
-
The effect of abatacept on T-cell activation is not long-lived in vivo.Discov Immunol. 2024 Jan 4;3(1):kyad029. doi: 10.1093/discim/kyad029. eCollection 2024. Discov Immunol. 2024. PMID: 38567291 Free PMC article.
-
Development and function of FOXP3+ regulators of immune responses.Clin Exp Immunol. 2023 Jul 5;213(1):13-22. doi: 10.1093/cei/uxad048. Clin Exp Immunol. 2023. PMID: 37085947 Free PMC article.
-
Regulatory T cells in autoimmune kidney diseases and transplantation.Nat Rev Nephrol. 2023 Sep;19(9):544-557. doi: 10.1038/s41581-023-00733-w. Epub 2023 Jul 3. Nat Rev Nephrol. 2023. PMID: 37400628 Review.
-
Current trends in sensitizing immune checkpoint inhibitors for cancer treatment.Mol Cancer. 2024 Dec 26;23(1):279. doi: 10.1186/s12943-024-02179-5. Mol Cancer. 2024. PMID: 39725966 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

