Finite element modeling of effects of tissue property variation on human optic nerve tethering during adduction
- PMID: 36347907
- PMCID: PMC9643519
- DOI: 10.1038/s41598-022-22899-2
Finite element modeling of effects of tissue property variation on human optic nerve tethering during adduction
Abstract
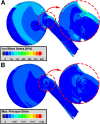
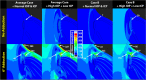
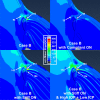
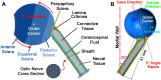
Tractional tethering by the optic nerve (ON) on the eye as it rotates towards the midline in adduction is a significant ocular mechanical load and has been suggested as a cause of ON damage induced by repetitive eye movements. We designed an ocular finite element model (FEM) simulating 6° incremental adduction beyond the initial configuration of 26° adduction that is the observed threshold for ON tethering. This FEM permitted sensitivity analysis of ON tethering using observed material property variations in measured hyperelasticity of the anterior, equatorial, posterior, and peripapillary sclera; and the ON and its sheath. The FEM predicted that adduction beyond the initiation of ON tethering concentrates stress and strain on the temporal side of the optic disc and peripapillary sclera, the ON sheath junction with the sclera, and retrolaminar ON neural tissue. However, some unfavorable combinations of tissue properties within the published ranges imposed higher stresses in these regions. With the least favorable combinations of tissue properties, adduction tethering was predicted to stress the ON junction and peripapillary sclera more than extreme conditions of intraocular and intracranial pressure. These simulations support the concept that ON tethering in adduction could induce mechanical stresses that might contribute to ON damage.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

