Developing an internal threshold of toxicological concern (iTTC)
- PMID: 36347933
- PMCID: PMC9731903
- DOI: 10.1038/s41370-022-00494-x
Developing an internal threshold of toxicological concern (iTTC)
Erratum in
-
Correction to: Developing an internal threshold of toxicological concern (iTTC).J Expo Sci Environ Epidemiol. 2023 Sep;33(5):842. doi: 10.1038/s41370-023-00582-6. J Expo Sci Environ Epidemiol. 2023. PMID: 37443298 Free PMC article. No abstract available.
Abstract
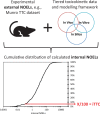
Background: Threshold of Toxicological Concern (TTC) approaches are used for chemical safety assessment and risk-based priority setting for data poor chemicals. TTCs are derived from in vivo No Observed Effect Level (NOEL) datasets involving an external administered dose from a single exposure route, e.g., oral intake rate. Thus, a route-specific TTC can only be compared to a route-specific exposure estimate and such TTCs cannot be used for other exposure scenarios such as aggregate exposures.
Objective: Develop and apply a method for deriving internal TTCs (iTTCs) that can be used in chemical assessments for multiple route-specific exposures (e.g., oral, inhalation or dermal) or aggregate exposures.
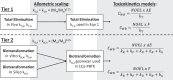
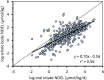
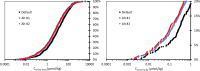
Methods: Chemical-specific toxicokinetics (TK) data and models are applied to calculate internal concentrations (whole-body and blood) from the reported administered oral dose NOELs used to derive the Munro TTCs. The new iTTCs are calculated from the 5th percentile of cumulative distributions of internal NOELs and the commonly applied uncertainty factor of 100 to extrapolate animal testing data for applications in human health assessment.
Results: The new iTTCs for whole-body and blood are 0.5 nmol/kg and 0.1 nmol/L, respectively. Because the iTTCs are expressed on a molar basis they are readily converted to chemical mass iTTCs using the molar mass of the chemical of interest. For example, the median molar mass in the dataset is 220 g/mol corresponding to an iTTC of 22 ng/L-blood (22 pg/mL-blood). The iTTCs are considered broadly applicable for many organic chemicals except those that are genotoxic or acetylcholinesterase inhibitors. The new iTTCs can be compared with measured or estimated whole-body or blood exposure concentrations for chemical safety screening and priority-setting.
Significance: Existing Threshold of Toxicological Concern (TTC) approaches are limited in their applications for route-specific exposure scenarios only and are not suitable for chemical risk and safety assessments under conditions of aggregate exposure. New internal Threshold of Toxicological Concern (iTTC) values are developed to address data gaps in chemical safety estimation for multi-route and aggregate exposures.
Keywords: Exposure modeling, Dermal exposure, Dietary exposure, Inhalation exposure, New approach methodologies (NAMs), PBPK modeling.
© 2022. The Author(s).
Conflict of interest statement
JAA, LT, JMA, AS, AL, TNB and LL acknowledge funding from the American Chemistry Council Long-Range Research Initiative (ACC-LRI). ARC Arnot Research and Consulting receives research funding from Health Canada, Environment and Climate Change Canada, and various chemical companies and industry groups. RB is a Senior Toxicologist and Director of the ACC-LRI at American Chemistry Council.
Figures

Comment in
-
Letter to the editor regarding recent publication titled "Developing an internal threshold of toxicological concern (iTTC)" by Arnot et al. (2022).J Expo Sci Environ Epidemiol. 2023 Sep;33(5):840-841. doi: 10.1038/s41370-023-00571-9. Epub 2023 Jul 14. J Expo Sci Environ Epidemiol. 2023. PMID: 37443297 No abstract available.
References
-
- U.S. EPA. Assessing and Managing Chemicals under TSCA; The Frank R. Lautenberg Chemical Safety for the 21st Century Act. Washington, DC. 2017.
-
- U.S. EPA. A Working Approach for Identifying Potential Candidate Chemicals for Prioritization. Washington, DC. 2018.
-
- Health Canada. Overview of the Chemicals Management Plan. Ottawa, ON. 2017.
-
- National Academies of Sciences Engineering, and Medicine. Using 21st Century Science to Improve Risk-Related Evaluations. The National Academies Press: Washington, DC. 2017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

