Mitotic Antipairing of Homologous Chromosomes
- PMID: 36348108
- PMCID: PMC9731508
- DOI: 10.1007/978-3-031-06573-6_6
Mitotic Antipairing of Homologous Chromosomes
Abstract
Chromosome organization is highly dynamic and plays an essential role during cell function. It was recently found that pairs of the homologous chromosomes are continuously separated at mitosis and display a haploid (1n) chromosome set, or "antipairing," organization in human cells. Here, we provide an introduction to the current knowledge of homologous antipairing in humans and its implications in human disease.
Keywords: Antipairing; Homologous chromosomes; Mitosis; Nuclear organization.
© 2022. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Figures


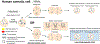
References
-
- Alcobia I, Dilao R, & Parreira L (2000). Spatial associations of centromeres in the nuclei of hematopoietic cells: Evidence for cell-type-specific organizational patterns. Blood, 95(5), 1608–1615. - PubMed
-
- Arnoldus EPJ, Noordermeer AI. A, Peters ACB, Raap AK, & Van der Ploeg M (1991). Interphase cytogenetics reveals somatic pairing of chromosome 17 centromeres in normal human brain tissue, but no trisomy 7 or sex-chromosome loss. Cytogenetics and Cell Genetics. - PubMed
-
- Atkin NB (1992). Observations on the pairing of homologous human nonacrocentric metaphase chromosomes. Chromatin, 105–109.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

