Sex differences in the medial prefrontal cortical glutamate system
- PMID: 36348414
- PMCID: PMC9641904
- DOI: 10.1186/s13293-022-00468-6
Sex differences in the medial prefrontal cortical glutamate system
Abstract
Background: Dysregulation in the prefrontal cortex underlies a variety of psychiatric illnesses, including substance use disorder, depression, and anxiety. Despite the established sex differences in prevalence and presentation of these illnesses, the neural mechanisms driving these differences are largely unexplored. Here, we investigate potential sex differences in glutamatergic transmission within the medial prefrontal cortex (mPFC). The goal of these experiments was to determine if there are baseline sex differences in transmission within this region that may underlie sex differences in diseases that involve dysregulation in the prefrontal cortex.
Methods: Adult male and female C57Bl/6J mice were used for all experiments. Mice were killed and bilateral tissue samples were taken from the medial prefrontal cortex for western blotting. Both synaptosomal and total GluA1 and GluA2 levels were measured. In a second set of experiments, mice were killed and ex vivo slice electrophysiology was performed on prepared tissue from the medial prefrontal cortex. Spontaneous excitatory postsynaptic currents and rectification indices were measured.
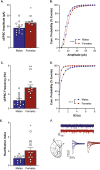
Results: Females exhibit higher levels of synaptosomal GluA1 and GluA2 in the mPFC compared to males. Despite similar trends, no statistically significant differences are seen in total levels of GluA1 and GluA2. Females also exhibit both a higher amplitude and higher frequency of spontaneous excitatory postsynaptic currents and greater inward rectification in the mPFC compared to males.
Conclusions: Overall, we conclude that there are sex differences in glutamatergic transmission in the mPFC. Our data suggest that females have higher levels of glutamatergic transmission in this region. This provides evidence that the development of sex-specific pharmacotherapies for various psychiatric diseases is important to create more effective treatments.
Keywords: AMPA receptors; Excitatory transmission; Glutamate; Medial prefrontal cortex; Sex differences.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

