Natural killer cells in clinical development as non-engineered, engineered, and combination therapies
- PMID: 36348457
- PMCID: PMC9644572
- DOI: 10.1186/s13045-022-01382-5
Natural killer cells in clinical development as non-engineered, engineered, and combination therapies
Abstract
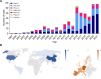
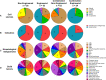
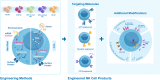
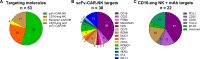
Natural killer (NK) cells are unique immune effectors able to kill cancer cells by direct recognition of surface ligands, without prior sensitization. Allogeneic NK transfer is a highly valuable treatment option for cancer and has recently emerged with hundreds of clinical trials paving the way to finally achieve market authorization. Advantages of NK cell therapies include the use of allogenic cell sources, off-the-shelf availability, and no risk of graft-versus-host disease (GvHD). Allogeneic NK cell therapies have reached the clinical stage as ex vivo expanded and differentiated non-engineered cells, as chimeric antigen receptor (CAR)-engineered or CD16-engineered products, or as combination therapies with antibodies, priming agents, and other drugs. This review summarizes the recent clinical status of allogeneic NK cell-based therapies for the treatment of hematological and solid tumors, discussing the main characteristics of the different cell sources used for NK product development, their use in cell manufacturing processes, the engineering methods and strategies adopted for genetically modified products, and the chosen approaches for combination therapies. A comparative analysis between NK-based non-engineered, engineered, and combination therapies is presented, examining the choices made by product developers regarding the NK cell source and the targeted tumor indications, for both solid and hematological cancers. Clinical trial outcomes are discussed and, when available, assessed in comparison with preclinical data. Regulatory challenges for product approval are reviewed, highlighting the lack of specificity of requirements and standardization between products. Additionally, the competitive landscape and business field is presented. This review offers a comprehensive overview of the effort driven by biotech and pharmaceutical companies and by academic centers to bring NK cell therapies to pivotal clinical trial stages and to market authorization.
Keywords: Adoptive cell therapy; Allogeneic; CAR-NK cells; Cancer; Combination therapy; GMP manufacturing; Genetic engineering; Immunotherapy; NK cell therapies; Natural killer cells; Off-the-shelf.
© 2022. The Author(s).
Conflict of interest statement
The authors are employees of Glycostem Therapeutics BV, a private company developing NK cell therapies.
Figures


References
-
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16(2):216–29. - PubMed
-
- Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–7. - PubMed
-
- Ortaldo JR, Oldham RK, Cannon GC, Herberman RB. Specificity of natural cytotoxic reactivity of normal human lymphocytes against a myeloid leukemia cell line. J Natl Cancer Inst. 1977;59(1):77–82. - PubMed
-
- Ljunggren HG, Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

