Preparation, characterization, and in-vitro cytotoxicity of nanoliposomes loaded with anti-tubercular drugs and TGF-β1 siRNA for improving spinal tuberculosis therapy
- PMID: 36348467
- PMCID: PMC9644586
- DOI: 10.1186/s12879-022-07791-8
Preparation, characterization, and in-vitro cytotoxicity of nanoliposomes loaded with anti-tubercular drugs and TGF-β1 siRNA for improving spinal tuberculosis therapy
Abstract
Background: Tuberculosis (TB) represents a bacterial infection affecting many individuals each year and potentially leading to death. Overexpression of transforming growth factor (TGF)-β1 has a primary immunomodulatory function in human tuberculosis. This work aimed to develop nanoliposomes to facilitate the delivery of anti-tubercular products to THP-1-derived human macrophages as Mycobacterium host cells and to evaluate drug efficiencies as well as the effects of a TGF-β1-specific short interfering RNA (siRNA) delivery system employing nanoliposomes.
Methods: In the current study, siTGF-β1 nanoliposomes loaded with the anti-TB drugs HRZ (isoniazid, rifampicin, and pyrazinamide) were prepared and characterized in vitro, determining the size, zeta potential, morphology, drug encapsulation efficiency (EE), cytotoxicity, and gene silencing efficiency of TGF-β1 siRNA.
Results: HRZ/siTGF-β1 nanoliposomes appeared as smooth spheres showing the size and positive zeta potential of 168.135 ± 0.5444 nm and + 4.03 ± 1.32 mV, respectively. Drug EEs were 90%, 88%, and 37% for INH, RIF, and PZA, respectively. Meanwhile, the nanoliposomes were weakly cytotoxic towards human macrophages as assessed by the MTT assay. Nanoliposomal siTGF-β1 could significantly downregulate TGF-β1 in THP-1-derived human macrophages in vitro.
Conclusion: These findings suggested that HRZ-loaded nanoliposomes with siTGF-β1 have the potential for improving spinal tuberculosis chemotherapy via nano-encapsulation of anti-TB drugs.
Keywords: Anti-tubercular drugs; Drug delivery; Nanoliposome; Spinal tuberculosis; TGF-β1 siRNA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

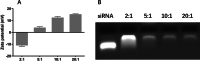






References
-
- Costa A, Pinheiro M, Magalhaes J, Ribeiro R, Seabra V, Reis S, et al. The formulation of nanomedicines for treating tuberculosis. Adv Drug Deliv Rev. 2016;102:102–115. - PubMed
-
- Broderick C, Hopkins S, Mack DJF, Aston W, Pollock R, Skinner JA, et al. Delays in the diagnosis and treatment of bone and joint tuberculosis in the United Kingdom. Bone Joint J. 2018;100-b(1):119–124. - PubMed
MeSH terms
Substances
Grants and funding
- grant no. 2020AAC03391/Ningxia Natural Science Foundation
- grant no. 2019-NW-011/the Autonomous Region health system research project
- 2022BEG03090/Key research and development projects in Ningxia Hui Autonomous Region
- grant no. 81501903/The present study was funded by the National Natural Science Foundation of China
- 81860395/The present study was funded by the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

