Association between the LRP1B and APOE loci and the development of Parkinson's disease dementia
- PMID: 36348503
- PMCID: PMC10151192
- DOI: 10.1093/brain/awac414
Association between the LRP1B and APOE loci and the development of Parkinson's disease dementia
Abstract
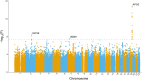
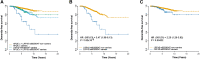
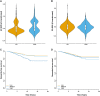
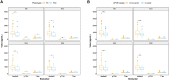
Parkinson's disease is one of the most common age-related neurodegenerative disorders. Although predominantly a motor disorder, cognitive impairment and dementia are important features of Parkinson's disease, particularly in the later stages of the disease. However, the rate of cognitive decline varies among Parkinson's disease patients, and the genetic basis for this heterogeneity is incompletely understood. To explore the genetic factors associated with rate of progression to Parkinson's disease dementia, we performed a genome-wide survival meta-analysis of 3923 clinically diagnosed Parkinson's disease cases of European ancestry from four longitudinal cohorts. In total, 6.7% of individuals with Parkinson's disease developed dementia during study follow-up, on average 4.4 ± 2.4 years from disease diagnosis. We have identified the APOE ε4 allele as a major risk factor for the conversion to Parkinson's disease dementia [hazard ratio = 2.41 (1.94-3.00), P = 2.32 × 10-15], as well as a new locus within the ApoE and APP receptor LRP1B gene [hazard ratio = 3.23 (2.17-4.81), P = 7.07 × 10-09]. In a candidate gene analysis, GBA variants were also identified to be associated with higher risk of progression to dementia [hazard ratio = 2.02 (1.21-3.32), P = 0.007]. CSF biomarker analysis also implicated the amyloid pathway in Parkinson's disease dementia, with significantly reduced levels of amyloid β42 (P = 0.0012) in Parkinson's disease dementia compared to Parkinson's disease without dementia. These results identify a new candidate gene associated with faster conversion to dementia in Parkinson's disease and suggest that amyloid-targeting therapy may have a role in preventing Parkinson's disease dementia.
Keywords: Parkinson’s disease; dementia; genome-wide; survival.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
H.R.M. reports paid consultancy from Roche. Research Grants from Parkinson’s UK, Cure Parkinson’s Trust, PSP Association, CBD Solutions, Drake Foundation, Medical Research Council (MRC), Michael J. Fox Foundation. H.R.M. is a co-applicant on a patent application related to C9ORF72—Method for diagnosing a neurodegenerative disease (PCT/GB2012/052140). D.G.G. has received grants from Michael’s Movers, the Neurosciences Foundation and Parkinson’s UK, and honoraria from AbbVie, BIAL Pharma, Britannia Pharmaceuticals, GE Healthcare and consultancy fees from Acorda Therapeutics and the Glasgow Memory Clinic. M.T.M.H. received funding/grant support from Parkinson’s UK, Oxford NIHR BRC, University of Oxford, CPT, Lab10X, NIHR, Michael J. Fox Foundation, H2020 European Union, GE Healthcare and the PSP Association. She also received payment for Advisory Board attendance/consultancy for Biogen, Roche, Sanofi, CuraSen Therapeutics, Evidera, Manus Neurodynamica, Lundbeck. Y.B.-S. has received grant funding from the MRC, NIHR, Parkinson’s UK, NIH and ESRC. J.C.C. has served on advisory boards for Biogen, Denali, Idorsia, Prevail Therapeutic, Servier, Theranexus, UCB and received grants from Sanofi and the Michael J. Fox Foundation outside of this work. A.E. received funding/grant support by Agence Nationale de la Recherche, France Parkinson and the Michael J. Fox Foundation. J.H. is supported by the UK Dementia Research Institute, which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. He is also supported by the MRC, Wellcome Trust, Dolby Family Fund, National Institute for Health Research University College London Hospitals Biomedical Research Centre. All other authors report no competing interests.
Figures

References
-
- Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124:901–905. - PubMed
-
- Dubois B, Burn D, Goetz C, et al. . Diagnostic procedures for Parkinson’s disease dementia: Recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. - PubMed
-
- Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology. 2008;70:1017–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

