Safety, immunogenicity & effectiveness of the COVID-19 vaccine among healthcare workers in a tertiary care hospital
- PMID: 36348600
- PMCID: PMC9807198
- DOI: 10.4103/ijmr.ijmr_1771_21
Safety, immunogenicity & effectiveness of the COVID-19 vaccine among healthcare workers in a tertiary care hospital
Abstract
Background & objectives: The COVID-19 pandemic has caused significant global morbidity and mortality. As the vaccination was rolled out with prioritization on healthcare workers (HCWs), it was desirable to generate evidence on effectiveness of vaccine in prevailing real-life situation for policy planning. The objective of the study was to evaluate the safety, effectiveness and immunogenicity of COVID-19 vaccination among HCWs in a tertiary care hospital.
Methods: This prospective observational study was undertaken on the safety, immunogenicity and effectiveness of the ChAdOx1 nCoV- 19 coronavirus vaccine (Recombinant) during the national vaccine roll out in January-March 2021, in a tertiary care hospital, New Delhi, India.
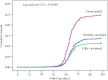
Results: The vaccine was found to be safe, with local pain, fever and headache as the most common adverse events of milder nature which generally lasted for two days. The adverse events following vaccination were lower in the second dose as compared to the first dose. The vaccine was immunogenic, with seropositivity, which was 51 per cent before vaccination, increasing to 77 per cent after single dose and 98 per cent after two doses. Subgroup analysis indicated that those with the past history of COVID-19 attained seropositivity of 98 per cent even with single dose. The incidence of reverse transcription (RT)-PCR positive COVID-19 was significantly lower among vaccinated (11.7%) as compared to unvaccinated (22.2%). Seven cases of moderate COVID-19 needing hospitalization were seen in the unvaccinated and only one such in the vaccinated group. The difference was significant between the fully vaccinated (10.8%) and the partially vaccinated (12.7%). The hazard of COVID-19 infection was higher among male, age >50 yr and clinical role in the hospital. After adjustment for these factors, the hazard of COVID-19 infection among unvaccinated was 2.09 as compared to fully vaccinated. Vaccine effectiveness was 52.2 per cent in HCWs.
Interpretation & conclusions: ChAdOx1 nCoV-19 coronavirus vaccine (Recombinant) was safe, immunogenic as well as showed effectiveness against the COVID-19 disease (CTRI/2021/01/030582).
Conflict of interest statement
Figures
References
-
- Kataria S, Sharma P, Deswal V, Kumar K, Singh MK, Alam S, et al. A real world evaluation of the safety and immunogenicity of the covishield vaccine, ChAdOx1 nCoV-19 corona virus vaccine (recombinant) in health care workers (HCW) in national capital region (NCR) of India:A preliminary report. medRxiv. 2021 Doi:10.1101/2021.04.14.21255452.
-
- DiaSorin. Liaison®SARS-CoV-2 S1/S2 IgG. The fully automated serology test for the detection of SARS-CoV-2 IgG antibody. [accessed on September 30, 2021]. Available from: https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov2... .
-
- Centers for Disease Control and Prevention. Lesson 3:Measures of risk. Section 6:Measures of public health impact. [accessed on September 30, 2021]. Available from: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html .
-
- Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Antibody response after first and second-dose of ChAdOx1-nCoV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India:The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492–6509. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous