Morphological Characterization of Astrocytes in a Xenograft of Human iPSCDerived Neural Precursor Cells
- PMID: 36348713
- PMCID: PMC9611864
- DOI: 10.32607/actanaturae.11710
Morphological Characterization of Astrocytes in a Xenograft of Human iPSCDerived Neural Precursor Cells
Abstract
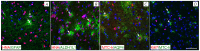
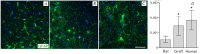
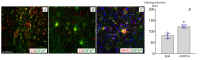
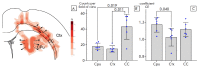
Transplantation of a mixed astrocyte and neuron culture is of interest in the development of cell therapies for neurodegenerative diseases. In this case, an assessment of engraftment requires a detailed morphological characterization, in particular an analysis of the neuronal and glial populations. In the experiment performed, human iPSC-derived neural progenitors transplanted into a rat striatum produced a mixed neuron and astrocyte population in vivo by the sixth month after transplantation. The morphological characteristics and neurochemical profile of the xenografted astrocytes were similar to those of mature human astroglia. Unlike neurons, astrocytes migrated to the surrounding structures and the density and pattern of their distribution in the striatum and cerebral cortex differed, which indicates that the microenvironment affects human glia integration. The graft was characterized by the zonal features of glial cell morphology, which was a reflection of cell maturation in the central area, glial shaft formation around the transplanted neurons, and migration to the surrounding structures.
Keywords: astrocytes; iPSC; neural precursors; striatum; transplantation.
Copyright ® 2022 National Research University Higher School of Economics.
Figures


References
-
- Bianchi F., Malboubi M., Li Y., George J.H., Jerusalem A., Szele F., Thompson M.S., Ye H.. Stem Cell Res. 2018;32:126–134. - PubMed
LinkOut - more resources
Full Text Sources
