An ELISA Platform for the Quantitative Analysis of SARS-CoV-2 RBD-neutralizing Antibodies As an Alternative to Monitoring of the Virus-Neutralizing Activity
- PMID: 36348715
- PMCID: PMC9611858
- DOI: 10.32607/actanaturae.11776
An ELISA Platform for the Quantitative Analysis of SARS-CoV-2 RBD-neutralizing Antibodies As an Alternative to Monitoring of the Virus-Neutralizing Activity
Abstract
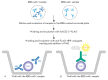
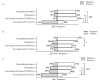
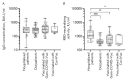
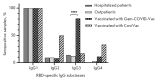
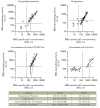
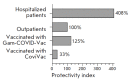
Monitoring of the level of the virus-neutralizing activity of serum immunoglobulins ensures that one can reliably assess the effectiveness of any protection against the SARS-CoV-2 infection. For SARS-CoV-2, the RBD-ACE2 neutralizing activity of sera is almost equivalent to the virus-neutralizing activity of their antibodies and can be used to assess the level of SARS-CoV-2 neutralizing antibodies. We are proposing an ELISA platform for performing a quantitative analysis of SARS-CoV-2 RBD-neutralizing antibodies, as an alternative to the monitoring of the virus-neutralizing activity using pseudovirus or "live" virus assays. The advantage of the developed platform is that it can be adapted to newly emerging virus variants in a very short time (1-2 weeks) and, thereby, provide quantitative data on the activity of SARS-CoV-2 RBD-neutralizing antibodies. The developed platform can be used to (1) study herd immunity to SARS-CoV-2, (2) monitor the effectiveness of the vaccination drive (revaccination) in a population, and (3) select potential donors of immune plasma. The protective properties of the humoral immune response in hospitalized patients and outpatients, as well as after prophylaxis with the two most popular SARS-CoV-2 vaccines in Russia, were studied in detail using this platform. The highest RBD-neutralizing activity was observed in the group of hospitalized patients. The protective effect in the group of individuals vaccinated with Gam-COVID-Vac vaccine was 25% higher than that in outpatients and almost four times higher than that in individuals vaccinated with the CoviVac vaccine.
Keywords: COVID-19; CoviVac; Gam-COVID-Vac; SARS-CoV-2; Sputnik V; antibodies; virus-neutralizing activity.
Copyright ® 2022 National Research University Higher School of Economics.
Figures
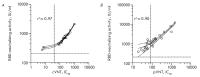
References
-
- https://covid19.who.int WHO Coronavirus (COVID-19) Dashboard.
-
- https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... WHO COVID-19 vaccine tracker and landscape.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
