Antagonism of nonaflatoxigenic Aspergillus flavus isolated from peanuts against aflatoxigenic A. flavus growth and aflatoxin B1 production in vitro
- PMID: 36348788
- PMCID: PMC9632215
- DOI: 10.1002/fsn3.2995
Antagonism of nonaflatoxigenic Aspergillus flavus isolated from peanuts against aflatoxigenic A. flavus growth and aflatoxin B1 production in vitro
Abstract
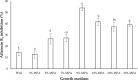
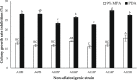
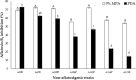
Aspergillus section Flavi constitutes several species of opportunistic fungi, notable among them are A. flavus and A. parasiticus, capable of surviving harsh conditions and colonizing a wide range of agricultural products pre- and postharvest. Physical and chemical control methods are widely applied in order to mitigate the invasion of A. flavus in crops. However, physical control is not suitable for large scale and chemical control often leads to environmental pollution, whereas biological control offers a safer, environmentally friendly, and economical alternative. The present study aimed to investigate the antagonism of several non-aflatoxigenic A. flavus strains against the aflatoxigenic ones in vitro (semisynthetic peanut growth medium; MPA) in terms of colony growth rate and AFB1 inhibition. Different peanut concentrations were used to obtain the optimum peanut concentration in the formulated growth medium. A dual culture assay was performed to assess the antagonism of nonaflatoxigenic strains against the aflatoxigenic ones. Results revealed that 9% MPA exhibited the highest growth and AFB1 inhibition by nonaflatoxigenic strains. It was also found that different nonaflatoxigenic strains exhibited different antagonism against the aflatoxigenic ones which ranged from 11.09 ± 0.65% to 14.06 ± 0.14% for growth inhibition, and 53.97 ± 2.46% to 72.64 ± 4.54% for AFB1 inhibition. This variability could be due to the difference in antagonistic metabolites produced by different nonaflatoxigenic strains assessed in the present study. Metabolomics study to ascertain the specific metabolites that conferred the growth and aflatoxin inhibition is ongoing.
Keywords: Aspergillus flavus; aflatoxigenic; aflatoxin B1; biocontrol; colony growth rate; non‐aflatoxigenic.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Impact of simulated climate change conditions on Aspergillus flavus biocontrol effectiveness in peanut-based medium and peanut seeds.Int J Food Microbiol. 2025 Jan 30;428:110981. doi: 10.1016/j.ijfoodmicro.2024.110981. Epub 2024 Nov 17. Int J Food Microbiol. 2025. PMID: 39579524
-
The role of extrolites secreted by nonaflatoxigenic Aspergillus flavus in biocontrol efficacy.J Appl Microbiol. 2019 Apr;126(4):1257-1264. doi: 10.1111/jam.14175. Epub 2019 Feb 7. J Appl Microbiol. 2019. PMID: 30548988
-
The effects of different packaging materials, temperatures and water activities to control aflatoxin B1 production by Aspergillus flavus and A. parasiticus in stored peanuts.J Food Sci Technol. 2019 Jun;56(6):3145-3150. doi: 10.1007/s13197-019-03652-6. Epub 2019 May 16. J Food Sci Technol. 2019. PMID: 31205369 Free PMC article.
-
Biological Control of Aflatoxin Contamination in U.S. Crops and the Use of Bioplastic Formulations of Aspergillus flavus Biocontrol Strains To Optimize Application Strategies.J Agric Food Chem. 2017 Aug 23;65(33):7081-7087. doi: 10.1021/acs.jafc.7b01452. Epub 2017 Apr 28. J Agric Food Chem. 2017. PMID: 28420231 Review.
-
Aspergillus section Flavi and Aflatoxins: Occurrence, Detection, and Identification in Raw Peanuts and Peanut-Based Products Along the Supply Chain.Front Microbiol. 2019 Nov 22;10:2602. doi: 10.3389/fmicb.2019.02602. eCollection 2019. Front Microbiol. 2019. PMID: 31824445 Free PMC article. Review.
Cited by
-
Occurrence of Aspergillus and Penicillium Species, Accumulation of Fungal Secondary Metabolites, and qPCR Detection of Potential Aflatoxigenic Aspergillus Species in Chickpea (Cicer arietinum L.) Seeds from Different Farming Systems.Foods. 2025 Jul 25;14(15):2610. doi: 10.3390/foods14152610. Foods. 2025. PMID: 40807547 Free PMC article.
-
Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking.Toxics. 2023 Sep 7;11(9):760. doi: 10.3390/toxics11090760. Toxics. 2023. PMID: 37755770 Free PMC article.
-
Mycotoxigenic fungal growth inhibition and multi-mycotoxin reduction of potential biological control agents indigenous to grain maize.Mycotoxin Res. 2023 Aug;39(3):177-192. doi: 10.1007/s12550-023-00484-4. Epub 2023 May 23. Mycotoxin Res. 2023. PMID: 37219742 Free PMC article.
-
Multi-omics characterization of aflatoxigenic Aspergillus from grains and rhizosphere of maize across agroecological zones of Cameroon.Sci Rep. 2025 May 26;15(1):18407. doi: 10.1038/s41598-025-97296-6. Sci Rep. 2025. PMID: 40419733 Free PMC article.
-
Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus.Toxins (Basel). 2024 Jul 12;16(7):314. doi: 10.3390/toxins16070314. Toxins (Basel). 2024. PMID: 39057954 Free PMC article.
References
-
- Ayelign, A. , & De Saeger, S. (2020). Mycotoxins in Ethiopia: Current status, implications to food safety and mitigation strategies. Food Control, 113, 107163.
-
- Bordin, K. , Sawada, M. M. , da Costa Rodrigues, C. E. , da Fonseca, C. R. , & Oliveira, C. A. F. (2014). Incidence of aflatoxins in oil seeds and possible transfer to oil: A review. Food Engineering Reviews, 6(1–2), 20–28.
-
- Chang, P. K. , Horn, B. W. , & Dorner, J. W. (2005). Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic aspergillus flavus isolates. Fungal Genetics and Biology, 42(11), 914–923. - PubMed
LinkOut - more resources
Full Text Sources

