Dietary aluminium intake disrupts the overall structure of gut microbiota in Wistar rats
- PMID: 36348807
- PMCID: PMC9632190
- DOI: 10.1002/fsn3.2955
Dietary aluminium intake disrupts the overall structure of gut microbiota in Wistar rats
Abstract
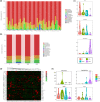
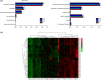
Approximately, 40% of ingested dietary aluminium accumulates in the intestine, which has been considered a target organ for dietary aluminium exposure. The gut microbiota may be the first protective barrier against the toxic metal aluminium and a crucial mediator of the bioavailability of metal aluminium. We previously evaluated dietary aluminium intake and its health risks in a population from Jilin Province, China, and found that the average daily intake of aluminium in the diet of residents in Jilin Province was 0.163 mg/kg after the total diet survey. In the present study, the equivalent concentration of aluminium in rats was extrapolated by the average dietary aluminium intake in the population of Jilin Province based on body surface area. Furthermore, healthy adult Wistar rats were randomly divided into four groups (n = 15 for each group): a control group and three groups treated with aluminium solution (1, 10, and 100 mg/kg/day, intragastrically) for 28 days. Following treatment, necrosis of renal tubular epithelial cells, hyperplasia of bile ducts and hyperplasia of heart tissue, as well as fiber in the liver, kidney, and heart tissues of aluminium-treated rats were observed, although there were no significant changes in the spleen and brain. Subsequently, fecal samples were withdrawn for 16S rRNA gene sequence analysis. It was found that aluminium decreased the microbiota diversity and changed the overall community structure of the gut microbiota, including three phyla and four genera, together with the regulation of 12 signaling pathways. Collectively, treatment with aluminium markedly altered the structure of the gut microbiota, suggesting that the disorders of intestinal flora induced by aluminium may be an important mechanism for aluminium toxicity.
Keywords: 16S rRNA sequencing; dietary aluminium; food safety; gut microbiome.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Fecal metabonomics combined with 16S rRNA gene sequencing to analyze the changes of gut microbiota in rats with kidney-yang deficiency syndrome and the intervention effect of You-gui pill.J Ethnopharmacol. 2019 Nov 15;244:112139. doi: 10.1016/j.jep.2019.112139. Epub 2019 Aug 8. J Ethnopharmacol. 2019. PMID: 31401318
-
[Revealing the role of gut microbiota in immune regulation and organ damage in sepsis using 16s rRNA and untargeted metabolomics].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023 Sep;35(9):927-932. doi: 10.3760/cma.j.cn121430-20230517-00378. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023. PMID: 37803951 Chinese.
-
Xylan alleviates dietary fiber deprivation-induced dysbiosis by selectively promoting Bifidobacterium pseudocatenulatum in pigs.Microbiome. 2021 Nov 21;9(1):227. doi: 10.1186/s40168-021-01175-x. Microbiome. 2021. PMID: 34802456 Free PMC article.
-
Genetic relationships between efficiency traits and gut microbiota traits in growing pigs being fed with a conventional or a high-fiber diet.J Anim Sci. 2022 Jun 1;100(6):skac183. doi: 10.1093/jas/skac183. J Anim Sci. 2022. PMID: 35579995 Free PMC article.
Cited by
-
Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index.Toxics. 2025 Jul 9;13(7):578. doi: 10.3390/toxics13070578. Toxics. 2025. PMID: 40711023 Free PMC article.
References
-
- Aguilar, F. , Autrup, H. , Barlow, S. , Castle, L. , & Toldrá, F. (2008). Safety of aluminium from dietary intake.
-
- Aluminium in food . (1994). Aluminium in food. Food and Chemical Toxicology, 32(4), 391–392. - PubMed
LinkOut - more resources
Full Text Sources

