Review on fluorinated nucleoside/non-nucleoside FDA-approved antiviral drugs
- PMID: 36348998
- PMCID: PMC9620415
- DOI: 10.1039/d2ra05370e
Review on fluorinated nucleoside/non-nucleoside FDA-approved antiviral drugs
Abstract
FDA-approved antiviral agents represent an important class that has attracted attention in recent years to combat current and future threats of viral pandemics. Fluorine ameliorates the electronic, lipophilic and steric problems of drugs. Additionally, fluorine can prolong drug activity and improve metabolic stability, thereby, modifying their pharmacodynamic and pharmacokinetic character. Herein, we summarized the fluorinated FDA-approved antiviral agents, dealing with biological aspects, mechanisms of action, and synthetic pathways.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

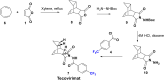
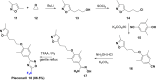
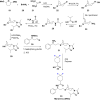

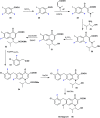


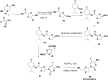

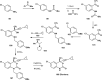
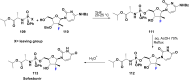
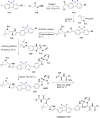
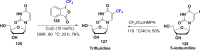
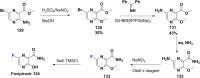
References
Publication types
LinkOut - more resources
Full Text Sources

