Influence of VO2 based structures and smart coatings on weather resistance for boosting the thermochromic properties of smart window applications
- PMID: 36349013
- PMCID: PMC9619487
- DOI: 10.1039/d2ra04661j
Influence of VO2 based structures and smart coatings on weather resistance for boosting the thermochromic properties of smart window applications
Abstract
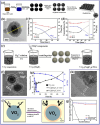
Vanadium dioxide (VO2)-based energy-saving smart films or coatings aroused great interest in scientific research and industry due to the reversible crystalline structural transition of VO2 from the monoclinic to tetragonal phase around room temperature, which can induce significant changes in transmittance and reflectance in the infrared (IR) range. However, there are still some obstacles for commercial application of VO2-based films or coatings in our daily life, such as the high phase transition temperature (68 °C), low luminous transmittance, solar modulation ability, and poor environmental stability. Particularly, due to its active nature chemically, VO2 is prone to gradual oxidation, causing deterioration of optical properties during very long life span of windows. In this review, the recent progress in enhancing the thermochromic properties of VO2-hybrid materials especially based on environmental stability has been summarized for the first time in terms of structural modifications such as core-shell structures for nanoparticles and nanorods and thin-films with single layer, layer-by-layer, and sandwich-like structures due to their excellent results for improving environmental stability. Moreover, future development trends have also been presented to promote the goal of commercial production of VO2 smart coatings.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
No conflict of interest.
Figures











Similar articles
-
Facile and Low-Temperature Fabrication of Thermochromic Cr2O3/VO2 Smart Coatings: Enhanced Solar Modulation Ability, High Luminous Transmittance and UV-Shielding Function.ACS Appl Mater Interfaces. 2017 Aug 9;9(31):26029-26037. doi: 10.1021/acsami.7b07137. Epub 2017 Jul 31. ACS Appl Mater Interfaces. 2017. PMID: 28723095
-
Phase-change VO2-based thermochromic smart windows.Light Sci Appl. 2024 Sep 18;13(1):255. doi: 10.1038/s41377-024-01560-9. Light Sci Appl. 2024. PMID: 39294120 Free PMC article. Review.
-
Core-shell VO2@TiO2 nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings.Sci Rep. 2013;3:1370. doi: 10.1038/srep01370. Sci Rep. 2013. PMID: 23546301 Free PMC article.
-
Infrared optical properties modulation of VO2 thin film fabricated by ultrafast pulsed laser deposition for thermochromic smart window applications.Sci Rep. 2022 Jul 6;12(1):11421. doi: 10.1038/s41598-022-15439-5. Sci Rep. 2022. PMID: 35794203 Free PMC article.
-
Thermochromic Vanadium Dioxide Nanostructures for Smart Windows and Radiative Cooling.Chemistry. 2024 Aug 1;30(43):e202400826. doi: 10.1002/chem.202400826. Epub 2024 Jul 12. Chemistry. 2024. PMID: 38818667 Review.
Cited by
-
Highly Tunable MOCVD Process of Vanadium Dioxide Thin Films: Relationship between Structural/Morphological Features and Electrodynamic Properties.Sensors (Basel). 2023 Aug 19;23(16):7270. doi: 10.3390/s23167270. Sensors (Basel). 2023. PMID: 37631806 Free PMC article.
-
Smart windows based on VO2 and WS2 monolayers.Nanoscale Adv. 2025 Jul 23. doi: 10.1039/d5na00584a. Online ahead of print. Nanoscale Adv. 2025. PMID: 40895583 Free PMC article.
References
-
- Granqvist C. G. Green S. Niklasson G. A. Mlyuka N. R. von Kræmer S. Georén P. Thin Solid Films. 2010;518:3046–3053.
-
- Granqvist C. G. Thin Solid Films. 2016;614:90–96.
-
- Granqvist C. G. Mater. Today: Proc. 2016;3:S2–S11.
-
- Wei G. Fan X. Xiong Y. Lv C. Li S. Lin X. Appl. Phys. Express. 2022;15(4):043002.
-
- Granqvist C. G. Lansåker P. C. Mlyuka N. R. Niklasson G. A. Avendaño E. Sol. Energy Mater. Sol. Cells. 2009;93:2032–2039.
Publication types
LinkOut - more resources
Full Text Sources

