Exploring inclusion complex of an anti-cancer drug (6-MP) with β-cyclodextrin and its binding with CT-DNA for innovative applications in anti-bacterial activity and photostability optimized by computational study
- PMID: 36349019
- PMCID: PMC9614615
- DOI: 10.1039/d2ra05072b
Exploring inclusion complex of an anti-cancer drug (6-MP) with β-cyclodextrin and its binding with CT-DNA for innovative applications in anti-bacterial activity and photostability optimized by computational study
Abstract
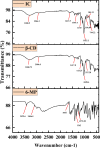
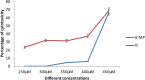
The co-evaporation approach was used to examine the host-guest interaction and to explore the cytotoxic and antibacterial properties of an important anti-cancer medication, 6-mercaptopurine monohydrate (6-MP) with β-cyclodextrin (β-CD). The UV-Vis investigation confirmed the inclusion complex's (IC) 1 : 1 stoichiometry and was also utilized to oversee the viability of this inclusion process. FTIR, NMR, and XRD, among other spectrometric techniques, revealed the mechanism of molecular interactions between β-CD and 6-MP which was further hypothesized by DFT to verify tentative outcomes. TGA and DSC studies revealed that 6-MP's thermal stability increased after encapsulation. Because of the protection of drug 6-MP by β-CD, the formed IC was found to have higher photostability. This work also predicts the release behavior of 6-MP in the presence of CT-DNA without any chemical changes. An evaluation of the complex's antibacterial activity in vitro revealed that it was more effective than pure 6-MP. The in vitro cytotoxic activity against the human kidney cancer cell line (ACHN) was also found to be significant for the IC (IC50 = 4.18 μM) compared to that of pure 6-MP (IC50 = 5.49 μM). These findings suggest that 6-MP incorporation via β-CD may result in 6-MP stability and effective presentation of its solubility, cytotoxic and antibacterial properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There were no known financial or personal conflicts of interest amongst the authors that could have influenced the work provided in this publication.
Figures















References
-
- Li H. Chen D.-X. Sun Y.-L. Zheng Y. B. Tan L.-L. Weiss P. S. Yang Y.-W. J. Am. Chem. Soc. 2013;135:1570–1576. - PubMed
-
- Negi J. S. Singh S. Carbohydr. Polym. 2013;92:1835–1843. - PubMed
-
- Strokopytov B. Penninga D. Rozeboom H. J. Kalk K. H. Dijkhuizen L. Dijkstra B. Biochem. 1995;34:2234–2240. - PubMed
-
- Cai L. Jeremic D. Lim H. Kim Y. Ind. Crops Prod. 2019;130:42–48.
-
- Bardi L. Mattei A. Steffan S. Marzona M. Enzyme Microb. Technol. 2000;27:709–713. - PubMed
LinkOut - more resources
Full Text Sources

