Pyridine ionic liquid functionalized bimetallic MOF solid-phase extraction coupled with high performance liquid chromatography for separation/analysis sunset yellow
- PMID: 36349023
- PMCID: PMC9614776
- DOI: 10.1039/d2ra05980k
Pyridine ionic liquid functionalized bimetallic MOF solid-phase extraction coupled with high performance liquid chromatography for separation/analysis sunset yellow
Abstract
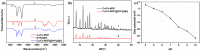
An effective method based on the pyridine ionic liquid functionalized bimetallic MOF solid-phase extractant (Cu/Co-MOF@[PrPy][Br]) coupled with high performance liquid chromatography (HPLC) for the separation/analysis sunset yellow was established. Cu/Co-MOF@[PrPy][Br] was characterized by FTIR, XRD, SEM and TEM. Several important factors, such as pH, amount of extractant, extract time, and types of eluents were investigated in detail. Under the optimal conditions, linear range of the method was 0.05-40.00 μg mL-1, the detection limit was 0.02 μg mL-1, and the linear correlation was good (R 2 = 0.9992). The analysis of sunset yellow in soda, effervescent tablet and jelly proved that the method was simple and effective.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare there is no conflicts of interest.
Figures




References
-
- Hosseininia S. A. R. Kamani M. H. Rani S. Quantitative determination of sunset yellow concentration in soft drinks via digital image processing. J. Food Meas. Char. 2017;11(3):1065–1070. doi: 10.1007/s11694-017-9483-8. - DOI
-
- Ji L. Cheng Q. Wu K. Yang X. F. Cu-BTC frameworks-based electrochemical sensing platform for rapid and simple determination of Sunset yellow and Tartrazine. Sens. Actuators, B. 2016;231:12–17. doi: 10.1016/j.snb.2016.03.012. - DOI
-
- Unsal Y. E. Soylak M. Tuzen M. Column solid-phase extraction of sunset yellow and spectrophotometric determination of its use in powdered beverage and confectionery products. Int. J. Food Sci. Technol. 2012;47(6):1253–1258. doi: 10.1111/j.1365-2621.2012.02966.x. - DOI
LinkOut - more resources
Full Text Sources

