Neurons derived from individual early Alzheimer's disease patients reflect their clinical vulnerability
- PMID: 36349119
- PMCID: PMC9636855
- DOI: 10.1093/braincomms/fcac267
Neurons derived from individual early Alzheimer's disease patients reflect their clinical vulnerability
Abstract
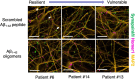
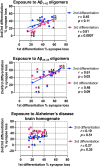
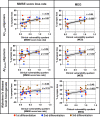
Establishing preclinical models of Alzheimer's disease that predict clinical outcomes remains a critically important, yet to date not fully realized, goal. Models derived from human cells offer considerable advantages over non-human models, including the potential to reflect some of the inter-individual differences that are apparent in patients. Here we report an approach using induced pluripotent stem cell-derived cortical neurons from people with early symptomatic Alzheimer's disease where we sought a match between individual disease characteristics in the cells with analogous characteristics in the people from whom they were derived. We show that the response to amyloid-β burden in life, as measured by cognitive decline and brain activity levels, varies between individuals and this vulnerability rating correlates with the individual cellular vulnerability to extrinsic amyloid-β in vitro as measured by synapse loss and function. Our findings indicate that patient-induced pluripotent stem cell-derived cortical neurons not only present key aspects of Alzheimer's disease pathology but also reflect key aspects of the clinical phenotypes of the same patients. Cellular models that reflect an individual's in-life clinical vulnerability thus represent a tractable method of Alzheimer's disease modelling using clinical data in combination with cellular phenotypes.
Keywords: Alzheimer’s disease; clinical vulnerability; disease modelling; induced pluripotent stem cells; synapse loss.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Prince M, Wimo A, Guerchet M, Gemma-Claire A, Wu Y-T, Prina M. World Alzheimer report 2015: The global impact of dementia—An analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int. 2015;84: Chapter 2; 10-29. 10.1111/j.0963-7214.2004.00293.x - DOI
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
Grants and funding
- MR/N029453/1/MRC_/Medical Research Council/United Kingdom
- K-1003/PUK_/Parkinson's UK/United Kingdom
- MR/T033371/1/MRC_/Medical Research Council/United Kingdom
- MR/N029941/1/MRC_/Medical Research Council/United Kingdom
- MR/P007058/1/MRC_/Medical Research Council/United Kingdom
- H-1102/PUK_/Parkinson's UK/United Kingdom
- H-1301/PUK_/Parkinson's UK/United Kingdom
- J-0901/PUK_/Parkinson's UK/United Kingdom
- G-0801/PUK_/Parkinson's UK/United Kingdom
- MC_EX_MR/N50192X/1/MRC_/Medical Research Council/United Kingdom
- MR/M024962/1/MRC_/Medical Research Council/United Kingdom
- G-1003/PUK_/Parkinson's UK/United Kingdom
- MC_U105597119/MRC_/Medical Research Council/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MC_UU_00005/12/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
