Alternative design strategies to help build the enzymatic retrosynthesis toolbox
- PMID: 36349225
- PMCID: PMC9627731
- DOI: 10.1039/d2cb00096b
Alternative design strategies to help build the enzymatic retrosynthesis toolbox
Abstract
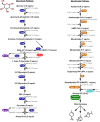
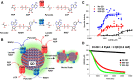
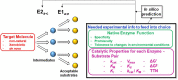
Most of the complex molecules found in nature still cannot be synthesized by current organic chemistry methods. Given the number of enzymes that exist in nature and the incredible potential of directed evolution, the field of synthetic biology contains perhaps all the necessary building blocks to bring about the realization of applied enzymatic retrosynthesis. Current thinking anticipates that enzymatic retrosynthesis will be implemented using conventional cell-based synthetic biology approaches where requisite native, heterologous, designer, and evolved enzymes making up a given multi-enzyme pathway are hosted by chassis organisms to carry out designer synthesis. In this perspective, we suggest that such an effort should not be limited by solely exploiting living cells and enzyme evolution and describe some useful yet less intensive complementary approaches that may prove especially productive in this grand scheme. By decoupling reactions from the environment of a living cell, a significantly larger portion of potential synthetic chemical space becomes available for exploration; most of this area is currently unavailable to cell-based approaches due to toxicity issues. In contrast, in a cell-free reaction a variety of classical enzymatic approaches can be exploited to improve performance and explore and understand a given enzyme's substrate specificity and catalytic profile towards non-natural substrates. We expect these studies will reveal unique enzymatic capabilities that are not accessible in living cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- Wöhler F. Ueber künstliche Bildung des Harnstoffs. Ann. Phys. 1828;88:253–256. doi: 10.1002/andp.18280880206. - DOI
-
- Corey E. J. Robert Robinson Lecture. Retrosynthetic Thinking—Essentials and Examples. Chem. Soc. Rev. 1988;17(0):111–133. doi: 10.1039/CS9881700111. - DOI
-
- Corey E. J., General Methods for the Construction of Complex Molecules, The Chemistry of Natural Products, Butterworth-Heinemann, Oxford, UK, 1967, pp. 19–37
-
- Jacob P. M. Lapkin A. Statistics of the Network of Organic Chemistry. React. Chem. Eng. 2018;3(1):102–118. doi: 10.1039/C7RE00129K. - DOI
Publication types
LinkOut - more resources
Full Text Sources

