Deciphering and reprogramming the cyclization regioselectivity in bifurcation of indole alkaloid biosynthesis
- PMID: 36349266
- PMCID: PMC9628931
- DOI: 10.1039/d2sc03612f
Deciphering and reprogramming the cyclization regioselectivity in bifurcation of indole alkaloid biosynthesis
Abstract
The metabolism of monoterpene indole alkaloids (MIAs) is an outstanding example of how plants shape chemical diversity from a single precursor. Here we report the discovery of novel enzymes from the Alstonia scholaris tree, a cytochrome P450, an NADPH dependent oxidoreductase and a BAHD acyltransferase that together synthesize the indole alkaloid akuammiline with a unique methanoquinolizidine cage structure. The two paralogous cytochrome P450 enzymes rhazimal synthase (AsRHS) and geissoschizine oxidase (AsGO) catalyse the cyclization of the common precursor geissoschizine and they direct the MIA metabolism towards to the two structurally distinct and medicinally important MIA classes of akuammilan and strychnos alkaloids, respectively. To understand the pathway divergence, we investigated the catalytic mechanism of the two P450 enzymes by homology modelling and reciprocal mutations. Upon conducting mutant enzyme assays, we identified a single amino acid residue that mediates the space in active sites, switches the enzymatic reaction outcome and impacts the cyclization regioselectivity. Our results represent a significant advance in MIA metabolism, paving the way for discovery of downstream genes in akuammilan alkaloid biosynthesis and facilitating future synthetic biology applications. We anticipate that our work presents, for the first time, insights at the molecular level for plant P450 catalytic activity with a significant key role in the diversification of alkaloid metabolism, and provides the basis for designing new drugs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


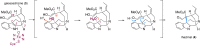
References
-
- Gaich T. Eckermann R. Synthesis. 2013;45:2813–2823. doi: 10.1055/s-0033-1339711. - DOI
-
- Smith J. M. Moreno J. Boal B. W. Garg N. K. Angew. Chem., Int. Ed. Engl. 2015;54:400–412. - PubMed
-
- Adams G. L. and Smith 3rd A. B., The Alkaloids: Chemistry and Biology, 2016, vol. 76, pp. 171–257 - PubMed
-
- Kearney S. E. Zahoránszky-Kőhalmi G. Brimacombe K. R. Henderson M. J. Lynch C. Zhao T. Wan K. K. Itkin Z. Dillon C. Shen M. Cheff D. M. Lee T. D. Bougie D. Cheng K. Coussens N. P. Dorjsuren D. Eastman R. T. Huang R. Iannotti M. J. Karavadhi S. Klumpp-Thomas C. Roth J. S. Sakamuru S. Sun W. Titus S. A. Yasgar A. Zhang Y.-Q. Zhao J. Andrade R. B. Brown M. K. Burns N. Z. Cha J. K. Mevers E. E. Clardy J. Clement J. A. Crooks P. A. Cuny G. D. Ganor J. Moreno J. Morrill L. A. Picazo E. Susick R. B. Garg N. K. Goess B. C. Grossman R. B. Hughes C. C. Johnston J. N. Joullie M. M. Kinghorn A. D. Kingston D. G. I. Krische M. J. Kwon O. Maimone T. J. Majumdar S. Maloney K. N. Mohamed E. Murphy B. T. Nagorny P. Olson D. E. Overman L. E. Brown L. E. Snyder J. K. Porco J. A. Rivas F. Ross S. A. Sarpong R. Sharma I. Shaw J. T. Xu Z. Shen B. Shi W. Stephenson C. R. J. Verano A. L. Tan D. S. Tang Y. ACS Cent. Sci. 2018;4:1727–1741. doi: 10.1021/acscentsci.8b00747. - DOI - PMC - PubMed
- Taylor R. E. Thomson R. J. Vosburg D. A. Wu J. Wuest W. M. Zakarian A. Zhang Y. Ren T. Zuo Z. Inglese J. Michael S. Simeonov A. Zheng W. Shinn P. Jadhav A. Boxer M. B. Hall M. D. Xia M. Guha R. Rohde J. M. ACS Cent. Sci. 2018;4:1727–1741. doi: 10.1021/acscentsci.8b00747. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

