Dysfunctional phenotype of systemic and pulmonary regulatory T cells associate with lethal COVID-19 cases
- PMID: 36349514
- PMCID: PMC9877711
- DOI: 10.1111/imm.13603
Dysfunctional phenotype of systemic and pulmonary regulatory T cells associate with lethal COVID-19 cases
Abstract
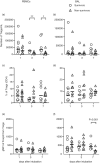
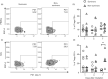
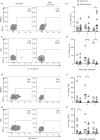
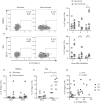
Severe cases of COVID-19 present hyperinflammatory condition that can be fatal. Little is known about the role of regulatory responses in SARS-CoV-2 infection. In this study, we evaluated the phenotype of regulatory T cells in the blood (peripheral blood mononuclear cell) and the lungs (broncho-alveolar) of adult patients with severe COVID-19 under invasive mechanical ventilation. Our results show important dynamic variation on Treg cells phenotype during COVID-19 with changes in number and functional parameters from the day of intubation (Day 1 of intensive care unit admission) to Day 7. We observed that compared with surviving patients, non-survivors presented lower numbers of Treg cells in the blood. In addition, lung Tregs of non-survivors also displayed higher PD1 and lower FOXP3 expressions suggesting dysfunctional phenotype. Further signs of Treg dysregulation were observed in non-survivors such as limited production of IL-10 in the lungs and higher production of IL-17A in the blood and in the lungs, which were associated with increased PD1 expression. These findings were also associated with lower pulmonary levels of Treg-stimulating factors like TNF and IL-2. Tregs in the blood and lungs are profoundly dysfunctional in non-surviving COVID-19 patients.
Keywords: COVID-19; invasive mechanical ventilation; regulatory T cells.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Coronavirus Resource Center, 2022. https://coronavirus.jhu.edu/data/mortality
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

