Kawasaki Disease Following the 13-valent Pneumococcal Conjugate Vaccine and Rotavirus Vaccines
- PMID: 36349537
- PMCID: PMC9724171
- DOI: 10.1542/peds.2022-058789
Kawasaki Disease Following the 13-valent Pneumococcal Conjugate Vaccine and Rotavirus Vaccines
Abstract
Background: Temporal associations between Kawasaki disease (KD) and childhood vaccines have been reported. Limited data on KD following 13-valent pneumococcal conjugate (PCV13) and rotavirus vaccines are available.
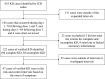
Methods: We conducted a self-controlled risk interval study using Vaccine Safety Datalink electronic health record data to investigate the risk of KD following PCV13 and rotavirus vaccines in children <2 years of age who were born from 2006 to 2017. All hospitalized KD cases identified by International Classification of Diseases diagnosis codes that fell within predefined risk (days 1-28 postvaccination) and control (days 29-56 for doses 1 and 2, and days 43-70 for doses 3 and 4) intervals were confirmed by manual chart review.
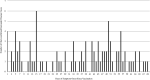
Results: During the study period, 655 cases of KD were identified by International Classification of Diseases codes. Of these, 97 chart-confirmed cases were within risk or control intervals. In analyses, the age-adjusted relative risk for KD following any dose of PCV13 was 0.75 (95% confidence interval, 0.47-1.21). Similarly, the age-adjusted relative risk for KD following any dose of rotavirus vaccine was 0.66 (95% CI, 0.40-1.09). Overall, there was no evidence of an elevated risk of KD following PCV13 or rotavirus vaccines by dose. In addition, no statistically significant temporal clustering of KD cases was identified during days 1 to 70 postvaccination.
Conclusions: PCV13 and rotavirus vaccination were not associated with an increased risk of KD in children <2 years of age. Our findings provide additional evidence for the overall safety of PCV13 and rotavirus vaccines.
Copyright © 2022 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
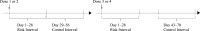
References
-
- McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999 - PubMed
-
- Shimada S, Watanabe T, Sato S. A patient with Kawasaki disease following influenza vaccinations. Pediatr Infect Dis J. 2015;34(8):913 - PubMed
-
- Kraszewska-Glomba B, Kuchar E, Szenborn L. Three episodes of Kawasaki disease including one after the Pneumo 23 vaccine in a child with a family history of Kawasaki disease. J Formos Med Assoc. 2016;115(10):885–886 - PubMed
-
- Miron D, Fink D, Hashkes PJ. Kawasaki disease in an infant following immunisation with hepatitis B vaccine. Clin Rheumatol. 2003;22(6):461–463 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

