Balloon Dilation of the Eustachian Tube: A Randomized Controlled Trial with 6 Months Follow-Up
- PMID: 36349672
- PMCID: PMC9682787
- DOI: 10.5152/iao.2022.21198
Balloon Dilation of the Eustachian Tube: A Randomized Controlled Trial with 6 Months Follow-Up
Abstract
Background: Obstructive Eustachian tube dysfunction in adults is common. The purpose of this study was to examine whether balloon dilation of the Eustachian tube can improve ventilation of the middle ear among adult patients with mild chronic Eustachian tube dysfunction.
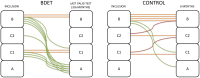
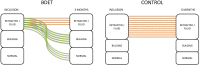
Methods: This study included patients aged ≥18 years with unilateral chronic Eustachian tube dysfunction confirmed with an abnormal tympanometry and a retracted tympanic membrane. Patients were treated daily with nasal steroid spray and Valsalva maneuver for 2 months. If Eustachian tube dysfunction persisted, they were enrolled in the study and randomized to balloon dilation of the Eustachian tube or control. All patients underwent otomicroscopy, tympanometry, pure-tone audiometry and the Eustachian Tube Dysfunction Questionnaire-7. Follow-up visits were completed at 3 weeks, 3 months, and 6 months.
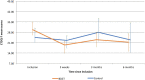
Results: In total, 24 patients completed the study (13 balloon dilation of the Eustachian tube, 11 control). The balloon dilation of the Eustachian tube group showed normalization from retraction or serous otitis media in 9 out of 13 patients (P = .0006) compared to 0 out of 11 patients in the control group. In the balloon dilation of the Eustachian tube group, 9 out of 13 patients showed an improvement in tympanometry from B to C/A or from C to A (P = .04) compared to 3 out of 11 patients in the control group. The audiometric data showed no difference (P = .38). There was no significant difference in mean Eustachian Tube Dysfunction Questionnaire-7 score between the two groups (P = .35). In the balloon dilation of the Eustachian tube group, 69% answered that they had benefitted from the treatment.
Conclusion: The procedure is feasible and no complications were reported. The study indicates that balloon dilation of the Eustachian tube may be a beneficial treatment in a selected group of adult patients with mild chronic Eustachian tube dysfunction.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
