2-hydroxy-1,4-naphthoquinones with 3-alkyldiarylether groups: synthesis and Plasmodium falciparum inhibitory activity
- PMID: 36349868
- PMCID: PMC9832320
- DOI: 10.4155/fmc-2022-0127
2-hydroxy-1,4-naphthoquinones with 3-alkyldiarylether groups: synthesis and Plasmodium falciparum inhibitory activity
Abstract
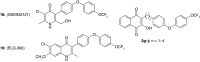
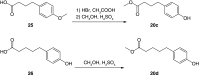
Background: In 1948, the synthesis and Plasmodium lophurae activity of 2-hydroxy-1,4-naphthoquinones containing 3-alkyldiarylether side chains was reported. Method/results: The synthesis of five related compounds, designed to be more metabolically stable, was pursued. The compounds were synthesized using a radical alkylation reaction with naphthoquinones. One compound had a lower IC50 value against various strains of Plasmodium falciparum and assay data indicate that it binds to the Qo site of cytochrome bc1. With a low yield for the radical alkylation of the most active compound, a reductive alkylation method with used to improve reaction yields. Conclusion: Further synthetic knowledge was obtained, and the assay data indicate that there are sensitivity differences between avian and human malarial parasites for these molecules.
Keywords: 2-hydroxy-1,4-naphthoquinones; Plasmodium falciparum; malaria; radical alkylation; three-component reductive alkylation.
Plain language summary
Malaria is a disease caused by a parasite that affects millions of people each year and results in many deaths. In 1948, 300 structurally related compounds were made and tested for antimalarial activity with the goal of finding a drug to treat the disease. From this work, promising compounds were identified and this work has served as a starting point for further investigations. Based on recent discoveries, this study made variations of promising 1948 compounds to investigate whether antimalarial activity could be improved. These compounds were made using two different methods. One derivative was found to be more potent than the original compound but was not the one expected based on the 1948 work.
Figures





References
-
- Fieser LF, Berliner E, Bondhus FJ et al. Naphthoquinone antimalarials. I. General survey. J. Am. Chem. Soc. 70(10), 3151–3155 (1948). - PubMed
-
•• General survey of Fieser's extensive naphthoquinone antimalarial research.
-
- Fieser LF, Berliner E, Bondhus FJ et al. Naphthoquinone antimalarials. IV-XI. Synthesis1. J. Am. Chem. Soc. 70(10), 3174–3215 (1948). - PubMed
-
• Describes a synthetic procedure to make the original compounds.
-
- Fieser LF, Heymann H. Naphthoquinone antimalarials: XXII. Relative antirespiratory activities (Plasmodium lophurae). J. Biol. Chem. 176(3), 1363–1370 (1948). - PubMed
-
- Fieser LF, Richardson AP. Naphthoquinone antimalarials. II. Correlation of structure and activity against P. lophurae in ducks. J. Am. Chem. Soc. 70(10), 3156–3165 (1948). - PubMed
-
- Fotie J. Quinones and malaria. Antiinfect. Agents Med. Chem. 5(4), 357–366 (2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
