Differences in the Reactivity of Geminal Si-O-P and Al-O-P Frustrated Lewis Pairs
- PMID: 36349870
- PMCID: PMC10107522
- DOI: 10.1002/chem.202202842
Differences in the Reactivity of Geminal Si-O-P and Al-O-P Frustrated Lewis Pairs
Abstract
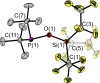
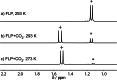
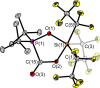
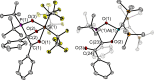
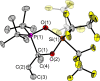
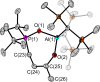
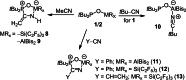
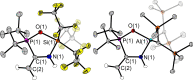
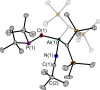
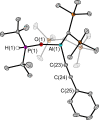
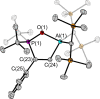
The new oxygen-bridged geminal Si/P Frustrated Lewis Pair (FLP) tBu2 P-O-Si(C2 F5 )3 (2) is able to reversibly bind carbon dioxide at ambient temperature. We compared its reactivity towards benzil, but-3-en-2-one, nitriles and phenylacetylene to that of the Al/P FLP tBu2 P-O-AlBis2 (Bis=-CH(SiMe3 )2 ) (1). When reacted with benzil, both, 1 and 2, form the 1,2-addition product, but in the Si/P FLP 2, the second carbonyl function additionally binds to the silicon atom. With but-3-en-2-one 2 forms the 1,2-addition product, while 1 binds in 1,4-position. The reaction with acetonitrile yielded an unexpected etheneimine adduct for both systems, while only 1 reacted with tert-butylnitrile. With benzonitrile and acrylonitrile, 2 showed reversible addition to the C≡N bond and 1 forms a stable adduct with benzonitrile. Solely 1 shows reactivity towards phenylacetylene affording a mixture of the CH deprotonation adduct tBu2 P(H)-O-AlBis2 (CCPh) and the FLP -C≡C 1,2-addition adduct under ring formation. All compounds were characterized by multinuclear NMR spectroscopy, XRD and elemental analysis.
Keywords: activation; aluminum; frustrated Lewis Pairs; silicon; small molecules.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
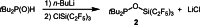
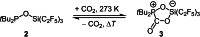
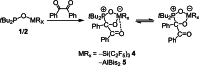
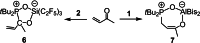
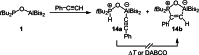
Similar articles
-
Reactivity of Oxygen-Bridged Geminal Al/P and Si/P Frustrated Lewis Pairs towards Heterocumulenes.Chemistry. 2023 Apr 13;29(21):e202203685. doi: 10.1002/chem.202203685. Epub 2023 Mar 9. Chemistry. 2023. PMID: 36734185
-
Selective 3,3-Rearrangement of Azobenzenes upon Complexation by a Frustrated Lewis Pair.Angew Chem Int Ed Engl. 2023 Mar 6;62(11):e202216943. doi: 10.1002/anie.202216943. Epub 2023 Feb 6. Angew Chem Int Ed Engl. 2023. PMID: 36645230
-
Carbon dioxide reduction by an Al-O-P frustrated Lewis pair.Chem Sci. 2022 Jun 2;13(27):8088-8094. doi: 10.1039/d2sc01870e. eCollection 2022 Jul 13. Chem Sci. 2022. PMID: 35919415 Free PMC article.
-
A P-H functionalized Al/P-based frustrated Lewis pair - hydrophosphination of nitriles, ring opening with cyclopropenones and evidence of P[double bond, length as m-dash]C double bond formation.Dalton Trans. 2018 Jun 25;47(25):8402-8417. doi: 10.1039/c8dt01836g. Dalton Trans. 2018. PMID: 29893387
-
Aluminum in Frustrated Lewis Pair Chemistry.Angew Chem Int Ed Engl. 2024 Aug 19;63(34):e202405207. doi: 10.1002/anie.202405207. Epub 2024 Jul 17. Angew Chem Int Ed Engl. 2024. PMID: 38826040 Review.
References
-
- None
-
- Welch G. C., San Juan R. R., Masuda J. D., Stephan D. W., Science 2006, 314, 1124; - PubMed
-
- Chase P. A., Welch G. C., Jurca T., Stephan D. W., Angew. Chem. Int. Ed. 2007, 46, 8050; - PubMed
-
- Welch G. C., Stephan D. W., J. Am. Chem. Soc. 2007, 129, 1880; - PubMed
-
- Spies P., Schwendemann S., Lange S., Kehr G., Fröhlich R., Erker G., Angew. Chem. Int. Ed. 2008, 47, 7543; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

