Cost-effectiveness of population screening for atrial fibrillation: the STROKESTOP study
- PMID: 36349968
- PMCID: PMC9839418
- DOI: 10.1093/eurheartj/ehac547
Cost-effectiveness of population screening for atrial fibrillation: the STROKESTOP study
Abstract
Aims: Previous studies on the cost-effectiveness of screening for atrial fibrillation (AF) are based on assumptions of long-term clinical effects. The STROKESTOP study, which randomised 27 975 persons aged 75/76 years into a screening invitation group and a control group, has a median follow-up time of 6.9 years. The aim of this study was to estimate the cost-effectiveness of population-based screening for AF using clinical outcomes.
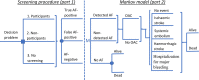
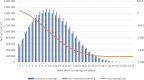
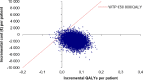
Methods and results: The analysis is based on a Markov cohort model. The prevalence of AF, the use of oral anticoagulation, clinical event data, and all-cause mortality were taken from the STROKESTOP study. The cost for clinical events, age-specific utilities, utility decrement due to stroke, and stroke death was taken from the literature. Uncertainty in the model was considered in a probabilistic sensitivity analysis. Per 1000 individuals invited to the screening, there were 77 gained life years and 65 gained quality-adjusted life years. The incremental cost was €1.77 million lower in the screening invitation group. Gained quality-adjusted life years to a lower cost means that the screening strategy was dominant. The result from 10 000 Monte Carlo simulations showed that the AF screening strategy was cost-effective in 99.2% and cost-saving in 92.7% of the simulations. In the base-case scenario, screening of 1000 individuals resulted in 10.6 [95% confidence interval (CI): -22.5 to 1.4] fewer strokes (8.4 ischaemic and 2.2 haemorrhagic strokes), 1.0 (95% CI: -1.9 to 4.1) more cases of systemic embolism, and 2.9 (95% CI: -18.2 to 13.1) fewer bleedings associated with hospitalization.
Conclusion: Based on the STROKESTOP study, this analysis shows that a broad AF screening strategy in an elderly population is cost-effective. Efforts should be made to increase screening participation.
Keywords: Atrial fibrillation; Cost-effectiveness; Markov modelling; Screening; Stroke prevention.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.L. has no conflicts of interest to report. E.S. has received institutional grants outside this work from Stockholm County Council (research position), Åke Wiberg Foundation, Swedish Heart Foundation, institutional consulting fees/payment honoraria for lectures/advisory board from Bayer, Bristol-Myers Squibb–Pfizer, Boehringer Ingelheim, Johnson & Johnson, Merck Sharp & Dohme, and is an unpaid European Heart Rhythm Association (EHRA) board member and chair of the digital committee (EHRA). L.B. has no conflicts of interest to report. M.A. was employed by AstraZeneca after the work was conducted. V.F. has received institutional grants or contracts from Medtronic, Abbott, and The Swedish Heart and Lung Foundation and payment for lectures from Medtronic. F.A-.K. has received consulting fees and payment or honoraria from lectures from Pfizer, Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, and sanofi-aventis. L.F. has received consulting fees from Bayer and Sanofi. M.R. received consulting fees from BMS-Pfizer, Roche, Zenicor, Medtronic, Janssen, payment or honoraria for lectures from Roche, BMS-Pfizer, support for attending meetings and/or travel from BMS-Pfizer, Medtronic, Roche, participation on advisory board for Medtronic SAE committee ICD, is a board member for Heart Runner Inc., and is the chairman for the Heart Foundation. J.E. has received grants or contracts from Roche Diagnostics, The Stockholm Region, Carl Bennet AB, The Swedish Heart & Lung Foundation, Swedish Research Foundation, Swedish Stroke Foundation, and Vinnova (Sweden’s Innovation Agency) and consulting fees from Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Roche Diagnostics, Philips, Piotrode, and Merck Sharp & Dome, and is a Delegate of the Swedish Ethical Review Authority. L.-—Å.L. has participated on a data safety monitoring board or advisory board for Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, and Bayer and owns stock in Astra Zeneca.
Figures
Comment in
-
Screening for atrial fibrillation to prevent stroke: increasing enthusiasm but outcomes still lag.Eur Heart J. 2023 Jan 14;44(3):205-207. doi: 10.1093/eurheartj/ehac696. Eur Heart J. 2023. PMID: 36458879 No abstract available.
Similar articles
-
Cost-effectiveness of Dabigatran Compared With Rivaroxaban for Prevention of Stroke and Systemic Embolism in Patients With Atrial Fibrillation in China.Clin Ther. 2020 Jan;42(1):144-156.e1. doi: 10.1016/j.clinthera.2019.11.011. Epub 2020 Jan 10. Clin Ther. 2020. PMID: 31932080
-
Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial.Lancet. 2021 Oct 23;398(10310):1498-1506. doi: 10.1016/S0140-6736(21)01637-8. Epub 2021 Aug 29. Lancet. 2021. PMID: 34469764 Clinical Trial.
-
Cost-Effectiveness of Extended and One-Time Screening Versus No Screening for Non-Valvular Atrial Fibrillation in the USA.Appl Health Econ Health Policy. 2020 Aug;18(4):533-545. doi: 10.1007/s40258-019-00542-y. Appl Health Econ Health Policy. 2020. PMID: 31849021 Free PMC article.
-
Review of economics and cost-effectiveness analyses of anticoagulant therapy for stroke prevention in atrial fibrillation in the US.Ann Pharmacother. 2013 May;47(5):671-85. doi: 10.1345/aph.1R411. Epub 2013 Apr 19. Ann Pharmacother. 2013. PMID: 23606551 Review.
-
Dabigatran for the prevention of stroke and systemic embolism in atrial fibrillation: A NICE single technology appraisal.Pharmacoeconomics. 2013 Jul;31(7):551-62. doi: 10.1007/s40273-013-0051-8. Pharmacoeconomics. 2013. PMID: 23620211 Review.
Cited by
-
Screening and diagnosis of atrial fibrillation using wearable devices.Korean J Intern Med. 2025 Jan;40(1):7-14. doi: 10.3904/kjim.2023.521. Epub 2024 May 3. Korean J Intern Med. 2025. PMID: 38699800 Free PMC article. Review.
-
Sinus node dysfunction and stroke risk: a systematic review and meta-analysis.BMJ Open. 2023 Nov 17;13(11):e076499. doi: 10.1136/bmjopen-2023-076499. BMJ Open. 2023. PMID: 37977871 Free PMC article.
-
Cost-Effectiveness of AF Screening With 2-Week Patch Monitors: The mSToPS Study.Circ Cardiovasc Qual Outcomes. 2023 Nov;16(11):e009751. doi: 10.1161/CIRCOUTCOMES.122.009751. Epub 2023 Oct 31. Circ Cardiovasc Qual Outcomes. 2023. PMID: 37905421 Free PMC article.
-
Yield of diagnosis and risk of stroke with screening strategies for atrial fibrillation: a comprehensive review of current evidence.Eur Heart J Open. 2023 Mar 22;3(2):oead031. doi: 10.1093/ehjopen/oead031. eCollection 2023 Mar. Eur Heart J Open. 2023. PMID: 37051263 Free PMC article. Review.
-
Atrial Fibrillation Screening in Asia: Balancing Costs and Benefits for Optimal Outcomes.JACC Asia. 2024 Dec 10;5(1):172-173. doi: 10.1016/j.jacasi.2024.10.009. eCollection 2025 Jan. JACC Asia. 2024. PMID: 39896254 Free PMC article.
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 - DOI - PubMed

