Chronic, episodic nicotine exposure alters GABAergic synaptic transmission to hypoglossal motor neurons and genioglossus muscle function at a critical developmental age
- PMID: 36350047
- PMCID: PMC9722256
- DOI: 10.1152/jn.00397.2022
Chronic, episodic nicotine exposure alters GABAergic synaptic transmission to hypoglossal motor neurons and genioglossus muscle function at a critical developmental age
Abstract
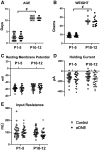
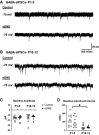
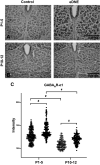
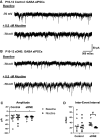
Regulation of GABAergic signaling through nicotinic acetylcholine receptor (nAChR) activation is critical for neuronal development. Here, we test the hypothesis that chronic episodic developmental nicotine exposure (eDNE) disrupts GABAergic signaling, leading to dysfunction of hypoglossal motor neurons (XIIMNs), which innervate the tongue muscles. We studied control and eDNE pups at two developmentally vulnerable age ranges: postnatal days (P)1-5 and P10-12. The amplitude and frequency of spontaneous and miniature inhibitory postsynaptic currents (sIPSCs, mIPSCs) at baseline were not altered by eDNE at either age. In contrast, eDNE increased GABAAR-α1 receptor expression on XIIMNs and, in the older group, the postsynaptic response to muscimol (GABAA receptor agonist). Activation of nAChRs with exogenous nicotine increased the frequency of GABAergic sIPSCs in control and eDNE neurons at P1-5. By P10-12, acute nicotine increased sIPSC frequency in eDNE but not control neurons. In vivo experiments showed that the breathing-related activation of tongue muscles, which are innervated by XIIMNs, is reduced at P10-12. This effect was partially mitigated by subcutaneous muscimol, but only in the eDNE pups. Taken together, these data indicate that eDNE alters GABAergic transmission to XIIMNs at a critical developmental age, and this is expressed as reduced breathing-related drive to XIIMNs in vivo.NEW & NOTEWORTHY Here, we provide a thorough assessment of the effects of nicotine exposure on GABAergic synaptic transmission, from the cellular to the systems level. This work makes significant advances in our understanding of the impact of nicotine exposure during development on GABAergic neurotransmission within the respiratory network and the potential role this plays in the excitatory/inhibitory imbalance that is thought to be an important mechanism underlying neonatal breathing disorders, including sudden infant death syndrome.
Keywords: GABA; development; hypoglossal; nicotine.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

