Effects of Renal Denervation vs Sham in Resistant Hypertension After Medication Escalation: Prespecified Analysis at 6 Months of the RADIANCE-HTN TRIO Randomized Clinical Trial
- PMID: 36350593
- PMCID: PMC9647563
- DOI: 10.1001/jamacardio.2022.3904
Effects of Renal Denervation vs Sham in Resistant Hypertension After Medication Escalation: Prespecified Analysis at 6 Months of the RADIANCE-HTN TRIO Randomized Clinical Trial
Abstract
Importance: Although early trials of endovascular renal denervation (RDN) for patients with resistant hypertension (RHTN) reported inconsistent results, ultrasound RDN (uRDN) was found to decrease blood pressure (BP) vs sham at 2 months in patients with RHTN taking stable background medications in the Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO) trial.
Objectives: To report the prespecified analysis of the persistence of the BP effects and safety of uRDN vs sham at 6 months in conjunction with escalating antihypertensive medications.
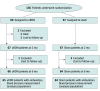
Design, setting, and participants: This randomized, sham-controlled, clinical trial with outcome assessors and patients blinded to treatment assignment, enrolled patients from March 11, 2016, to March 13, 2020. This was an international, multicenter study conducted in the US and Europe. Participants with daytime ambulatory BP of 135/85 mm Hg or higher after 4 weeks of single-pill triple-combination treatment (angiotensin-receptor blocker, calcium channel blocker, and thiazide diuretic) with estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73 m2 or greater were randomly assigned to uRDN or sham with medications unchanged through 2 months. From 2 to 5 months, if monthly home BP was 135/85 mm Hg or higher, standardized stepped-care antihypertensive treatment starting with aldosterone antagonists was initiated under blinding to treatment assignment.
Interventions: uRDN vs sham procedure in conjunction with added medications to target BP control.
Main outcomes and measures: Six-month change in medications, change in daytime ambulatory systolic BP, change in home systolic BP adjusted for baseline BP and medications, and safety.
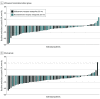
Results: A total of 65 of 69 participants in the uRDN group and 64 of 67 participants in the sham group (mean [SD] age, 52.4 [8.3] years; 104 male [80.6%]) with a mean (SD) eGFR of 81.5 (22.8) mL/min/1.73 m2 had 6-month daytime ambulatory BP measurements. Fewer medications were added in the uRDN group (mean [SD], 0.7 [1.0] medications) vs sham (mean [SD], 1.1 [1.1] medications; P = .045) and fewer patients in the uRDN group received aldosterone antagonists at 6 months (26 of 65 [40.0%] vs 39 of 64 [60.9%]; P = .02). Despite less intensive standardized stepped-care antihypertensive treatment, mean (SD) daytime ambulatory BP at 6 months was 138.3 (15.1) mm Hg with uRDN vs 139.0 (14.3) mm Hg with sham (additional decreases of -2.4 [16.6] vs -7.0 [16.7] mm Hg from month 2, respectively), whereas home SBP was lowered to a greater extent with uRDN by 4.3 mm Hg (95% CI, 0.5-8.1 mm Hg; P = .03) in a mixed model adjusting for baseline and number of medications. Adverse events were infrequent and similar between groups.
Conclusions and relevance: In this study, in patients with RHTN initially randomly assigned to uRDN or a sham procedure and who had persistent elevation of BP at 2 months after the procedure, standardized stepped-care antihypertensive treatment escalation resulted in similar BP reduction in both groups at 6 months, with fewer additional medications required in the uRDN group.
Trial registration: ClinicalTrials.gov Identifier: NCT02649426.
Conflict of interest statement
Figures

References
-
- Azizi M, Sapoval M, Gosse P, et al. ; Renal Denervation for Hypertension (DENERHTN) investigators . Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957-1965. doi:10.1016/S0140-6736(14)61942-5 - DOI - PubMed
-
- Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335-2345. doi:10.1016/S0140-6736(18)31082-1 - DOI - PubMed
-
- Böhm M, Kario K, Kandzari DE, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators . Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444-1451. doi:10.1016/S0140-6736(20)30554-7 - DOI - PubMed
-
- Kandzari DE, Böhm M, Mahfoud F, et al. ; SPYRAL HTN-ON MED Trial Investigators . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346-2355. doi:10.1016/S0140-6736(18)30951-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

