Oral mitis group streptococci reduce infectivity of influenza A virus via acidification and H2O2 production
- PMID: 36350830
- PMCID: PMC9645635
- DOI: 10.1371/journal.pone.0276293
Oral mitis group streptococci reduce infectivity of influenza A virus via acidification and H2O2 production
Abstract
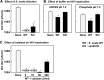
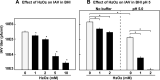
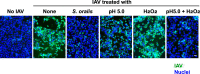
Members of the mitis group streptococci are the most abundant inhabitants of the oral cavity and dental plaque. Influenza A virus (IAV), the causative agent of influenza, infects the upper respiratory tract, and co-infection with Streptococcus pneumoniae is a major cause of morbidity during influenza epidemics. S. pneumoniae is a member of mitis group streptococci and shares many features with oral mitis group streptococci. In this study, we investigated the effect of viable Streptococcus oralis, a representative member of oral mitis group, on the infectivity of H1N1 IAV. The infectivity of IAV was measured by a plaque assay using Madin-Darby canine kidney cells. When IAV was incubated in growing culture of S. oralis, the IAV titer decreased in a time- and dose-dependent manner and became less than 100-fold, whereas heat-inactivated S. oralis had no effect. Other oral streptococci such as Streptococcus mutans and Streptococcus salivarius also reduced the viral infectivity to a lesser extent compared to S. oralis and Streptococcus gordonii, another member of the oral mitis group. S. oralis produces hydrogen peroxide (H2O2) at a concentration of 1-2 mM, and its mutant deficient in H2O2 production showed a weaker effect on the inactivation of IAV, suggesting that H2O2 contributes to viral inactivation. The contribution of H2O2 was confirmed by an inhibition assay using catalase, an H2O2-decomposing enzyme. These oral streptococci produce short chain fatty acids (SCFA) such as acetic acid as a by-product of sugar metabolism, and we also found that the inactivation of IAV was dependent on the mildly acidic pH (around pH 5.0) of these streptococcal cultures. Although inactivation of IAV in buffers of pH 5.0 was limited, incubation in the same buffer containing 2 mM H2O2 resulted in marked inactivation of IAV, which was similar to the effect of growing S. oralis culture. Taken together, these results reveal that viable S. oralis can inactivate IAV via the production of SCFAs and H2O2. This finding also suggests that the combination of mildly acidic pH and H2O2 at low concentrations could be an effective method to inactivate IAV.
Copyright: © 2022 Okahashi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
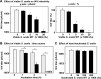
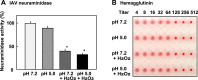
 : with S. oralis;
: with S. oralis;  : without S. oralis. The data are shown as mean ± SD values of triplicate samples. *p < 0.05 as compared with the untreated control (no bacteria, or no S. oralis).
: without S. oralis. The data are shown as mean ± SD values of triplicate samples. *p < 0.05 as compared with the untreated control (no bacteria, or no S. oralis).


Similar articles
-
Oral mitis group streptococci: A silent majority in our oral cavity.Microbiol Immunol. 2022 Dec;66(12):539-551. doi: 10.1111/1348-0421.13028. Epub 2022 Oct 4. Microbiol Immunol. 2022. PMID: 36114681 Review.
-
Hydrogen peroxide contributes to the epithelial cell death induced by the oral mitis group of streptococci.PLoS One. 2014 Jan 31;9(1):e88136. doi: 10.1371/journal.pone.0088136. eCollection 2014. PLoS One. 2014. PMID: 24498253 Free PMC article.
-
The activation of the oxidative stress response transcription factor SKN-1 in Caenorhabditis elegans by mitis group streptococci.PLoS One. 2018 Aug 16;13(8):e0202233. doi: 10.1371/journal.pone.0202233. eCollection 2018. PLoS One. 2018. PMID: 30114261 Free PMC article.
-
Microarray analysis of macrophage response to infection with Streptococcus oralis reveals the immunosuppressive effect of hydrogen peroxide.Biochem Biophys Res Commun. 2017 Apr 1;485(2):461-467. doi: 10.1016/j.bbrc.2017.02.048. Epub 2017 Feb 13. Biochem Biophys Res Commun. 2017. PMID: 28202416
-
Secondary streptococcal infection following influenza.Microbiol Immunol. 2022 Jun;66(6):253-263. doi: 10.1111/1348-0421.12965. Epub 2022 May 26. Microbiol Immunol. 2022. PMID: 35088451 Review.
Cited by
-
Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 17: suitability of taxonomic units notified to EFSA until September 2022.EFSA J. 2023 Jan 25;21(1):e07746. doi: 10.2903/j.efsa.2023.7746. eCollection 2023 Jan. EFSA J. 2023. PMID: 36704192 Free PMC article.
-
Respiratory tract barrier dysfunction in viral-bacterial co-infection cases.Jpn Dent Sci Rev. 2024 Dec;60:44-52. doi: 10.1016/j.jdsr.2023.12.006. Epub 2024 Jan 6. Jpn Dent Sci Rev. 2024. PMID: 38274948 Free PMC article. Review.
References
-
- Public Health England. Pyogenic and non-pyogenic streptococcal bacteraemia in England, Wales and Northern Ireland. Health Protection Reports [serial online]. 2013; 8: No.44. (doi is not appeared).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

