Synthesis of Stereoenriched Piperidines via Chemo-Enzymatic Dearomatization of Activated Pyridines
- PMID: 36350999
- PMCID: PMC9706572
- DOI: 10.1021/jacs.2c07143
Synthesis of Stereoenriched Piperidines via Chemo-Enzymatic Dearomatization of Activated Pyridines
Abstract
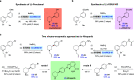
The development of efficient and sustainable methods for the synthesis of nitrogen heterocycles is an important goal for the chemical industry. In particular, substituted chiral piperidines are prominent targets due to their prevalence in medicinally relevant compounds and their precursors. A potential biocatalytic approach to the synthesis of this privileged scaffold would be the asymmetric dearomatization of readily assembled activated pyridines. However, nature is yet to yield a suitable biocatalyst specifically for this reaction. Here, by combining chemical synthesis and biocatalysis, we present a general chemo-enzymatic approach for the asymmetric dearomatization of activated pyridines for the preparation of substituted piperidines with precise stereochemistry. The key step involves a stereoselective one-pot amine oxidase/ene imine reductase cascade to convert N-substituted tetrahydropyridines to stereo-defined 3- and 3,4-substituted piperidines. This chemo-enzymatic approach has proved useful for key transformations in the syntheses of antipsychotic drugs Preclamol and OSU-6162, as well as for the preparation of two important intermediates in synthetic routes of the ovarian cancer monotherapeutic Niraparib.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Joule J. A. Natural Products Containing Nitrogen Heterocycles—Some Highlights 1990–2015. In Advances in Heterocyclic Chemistry; Elsevier Ltd, 2016; Vol. 119, pp. 81–106.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

