Cryo-EM structures of peripherin-2 and ROM1 suggest multiple roles in photoreceptor membrane morphogenesis
- PMID: 36351012
- PMCID: PMC9645710
- DOI: 10.1126/sciadv.add3677
Cryo-EM structures of peripherin-2 and ROM1 suggest multiple roles in photoreceptor membrane morphogenesis
Abstract
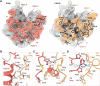
Mammalian peripherin-2 (PRPH2) and rod outer segment membrane protein 1 (ROM1) are retina-specific tetraspanins that partake in the constant renewal of stacked membrane discs of photoreceptor cells that enable vision. Here, we present single-particle cryo-electron microscopy structures of solubilized PRPH2-ROM1 heterodimers and higher-order oligomers. High-risk PRPH2 and ROM1 mutations causing blindness map to the protein-dimer interface. Cysteine bridges connect dimers forming positive-curved oligomers, whereas negative-curved oligomers were observed occasionally. Hexamers and octamers exhibit a secondary micelle that envelopes four carboxyl-terminal helices, supporting a potential role in membrane remodeling. Together, the data indicate multiple structures for PRPH2-ROM1 in creating and maintaining compartmentalization of photoreceptor cells.
Figures



References
-
- Goldberg A. F. X., Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int. Rev. Cytol. 253, 131–175 (2006). - PubMed
-
- Steinberg R. H., Fisher S. K., Anderson D. H., Disc morphogenesis in vertebrate photoreceptors. J. Comp. Neurol. 190, 501–518 (1980). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

