Evaluating lleal Pouch Anal Anastomosis Function: Time to Expand Our ARM-amentarium
- PMID: 36351035
- PMCID: PMC11007395
- DOI: 10.1093/ibd/izac234
Evaluating lleal Pouch Anal Anastomosis Function: Time to Expand Our ARM-amentarium
Abstract
Background: Total proctocolectomy with ileal pouch anal anastomosis (IPAA) for medically refractory ulcerative colitis or dysplasia may be associated with structural and inflammatory complications. However, even in their absence, defecatory symptoms secondary to dyssynergic defecation or fecal incontinence may occur. Although anorectal manometry is well established as the diagnostic test of choice for defecatory symptoms, its utility in the assessment of patients with IPAA is less established. In this systematic review, we critically evaluate the existing evidence for anopouch manometry (APM).
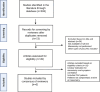
Methods: A total of 393 studies were identified, of which 6 studies met all inclusion criteria. Studies were not pooled given different modalities of testing with varying outcome measures.
Results: Overall, less than 10% of symptomatic patients post-IPAA were referred to APM. The prevalence of dyssynergic defecation as defined by the Rome IV criteria in symptomatic patients with IPAA ranged from 47.0% to 100%. Fecal incontinence in patients with IPAA was characterized by decreased mean and maximal resting anal pressure on APM, as well as pouch hyposensitivity. The recto-anal inhibitory reflex was absent in most patients with and without incontinence.
Conclusion: Manometry alone is an imperfect assessment of pouch function in patients with defecatory symptoms, and confirmatory testing may need to be performed with dynamic imaging.
Keywords: ileal pouch anal anastomosis; manometry.
Plain language summary
Dyssynergic defecation and fecal incontinence are increasingly being recognized in symptomatic patients with ileal pouch anal anastomosis. Manometry alone is an imperfect assessment of pouch function in patients with defecatory symptoms, and confirmatory testing may need to be performed with dynamic imaging.
© The Author(s) 2022. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Y.L. has received consulting fees from Mahana Therapeutics. M.K. has served as a consultant for GoodRx and receives grant support from NIH-DK127241-01A1. M.C.D. has served as a consultant for Abbvie, Arena Pharmaceuticals, Boehringer Ingelheim International, Bristol-Myers Squibb, Celgene, Eli Lilly, F. Hoffman-La Roche, Genentech, Gilead, Janssen, Pfizer, Prometheus, Takeda, UCB, has research grants from Pfizer, Abbvie, Janssen, and Prometheus, has ownership interest in Trellus and licensing fees from Takeda. S.M. reports receiving research grants from Genentech and Takeda; receiving payment for lectures from Takeda, Genentech, Morphic; and receiving consulting fees from Takeda, Morphic, Ferring, and Arena Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous