The Development and Consequences of Red Blood Cell Alloimmunization
- PMID: 36351365
- PMCID: PMC10414795
- DOI: 10.1146/annurev-pathol-042320-110411
The Development and Consequences of Red Blood Cell Alloimmunization
Abstract
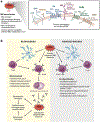
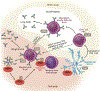
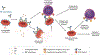
While red blood cell (RBC) transfusion is the most common medical intervention in hospitalized patients, as with any therapeutic, it is not without risk. Allogeneic RBC exposure can result in recipient alloimmunization, which can limit the availability of compatible RBCs for future transfusions and increase the risk of transfusion complications. Despite these challenges and the discovery of RBC alloantigens more than a century ago, relatively little has historically been known regarding the immune factors that regulate RBC alloantibody formation. Through recent epidemiological approaches, in vitro-based translational studies, and newly developed preclinical models, the processes that govern RBC alloimmunization have emerged as more complex and intriguing than previously appreciated. Although common alloimmunization mechanisms exist, distinct immune pathways can be engaged, depending on the target alloantigen involved. Despite this complexity, key themes are beginning to emerge that may provide promising approaches to not only actively prevent but also possibly alleviate the most severe complications of RBC alloimmunization.
Keywords: alloimmunization; hemolysis; red blood cell; sickle cell disease; transfusion medicine.
Figures

Similar articles
-
The ongoing challenge of RBC alloimmunization in the management of patients with sickle cell disease.Presse Med. 2023 Dec;52(4):104211. doi: 10.1016/j.lpm.2023.104211. Epub 2023 Nov 17. Presse Med. 2023. PMID: 37981194
-
Transfusion-related red blood cell alloantibodies: induction and consequences.Blood. 2019 Apr 25;133(17):1821-1830. doi: 10.1182/blood-2018-08-833962. Epub 2019 Feb 26. Blood. 2019. PMID: 30808636 Free PMC article. Review.
-
Impact of Red Blood Cell Antigen Matching on Alloimmunization and Transfusion Complications in Patients with Sickle Cell Disease: A Systematic Review.Transfus Med Rev. 2019 Jan;33(1):12-23. doi: 10.1016/j.tmrv.2018.07.003. Epub 2018 Jul 26. Transfus Med Rev. 2019. PMID: 30122266
-
Clodronate inhibits alloimmunization against distinct red blood cell alloantigens in mice.Transfusion. 2022 May;62(5):948-953. doi: 10.1111/trf.16872. Epub 2022 Apr 26. Transfusion. 2022. PMID: 35470900 Free PMC article.
-
Associations between blood donors, component modifications, and the alloimmunization of transfusion recipients.Transfusion. 2025 Mar;65(3):588-603. doi: 10.1111/trf.18135. Epub 2025 Jan 17. Transfusion. 2025. PMID: 39821794
Cited by
-
Prior immunization against an intracellular antigen enhances subsequent red blood cell alloimmunization in mice.Blood. 2023 May 25;141(21):2642-2653. doi: 10.1182/blood.2022016588. Blood. 2023. PMID: 36638335 Free PMC article.
-
Eleven years of alloimmunization in 6496 patients with sickle cell disease in France who received transfusion.Blood Adv. 2023 Dec 26;7(24):7608-7620. doi: 10.1182/bloodadvances.2022009328. Blood Adv. 2023. PMID: 37699002 Free PMC article.
-
Whole microbe arrays accurately predict interactions and overall antimicrobial activity of galectin-8 toward distinct strains of Streptococcus pneumoniae.Sci Rep. 2023 Apr 1;13(1):5324. doi: 10.1038/s41598-023-27964-y. Sci Rep. 2023. PMID: 37005394 Free PMC article.
-
The Nrf2 Activator CDDO-Imidazole Suppresses Inflammation-Induced Red Blood Cell Alloimmunization.Antioxidants (Basel). 2025 Jun 3;14(6):678. doi: 10.3390/antiox14060678. Antioxidants (Basel). 2025. PMID: 40563311 Free PMC article.
-
ABO blood groups and galectins: Implications in transfusion medicine and innate immunity.Semin Immunol. 2024 Jul-Sep;74-75:101892. doi: 10.1016/j.smim.2024.101892. Epub 2024 Oct 14. Semin Immunol. 2024. PMID: 39405833 Review.
References
-
- Denis J 1668. An extract of a letter, written by J. Denis, doctor of physick, and professor of philosophy and the mathematicks at Paris, touching a late cure of an inveterate phrensy by the transfusion of bloud. Philos. Trans. R. Soc. Lond 2:617–24
-
- Greenwalt T 1997. A short history of transfusion medicine. Transfusion 37:550–63 - PubMed
-
- Landsteiner K 1900. Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe. Zentralbl. Bakteriol 27:357–62
-
- Stowell CP, Stowell SR. 2019. Biologic roles of the ABH and Lewis histo-blood group antigens part I: infection and immunity. Vox Sang. 114:426–42 - PubMed
-
- Stowell SR, Stowell CP. 2019. Biologic roles of the ABH and Lewis histo-blood group antigens part II: thrombosis, cardiovascular disease and metabolism. Vox Sang. 114:535–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

