Sex differences in resilience to ferroptosis underlie sexual dimorphism in kidney injury and repair
- PMID: 36351395
- PMCID: PMC9795409
- DOI: 10.1016/j.celrep.2022.111610
Sex differences in resilience to ferroptosis underlie sexual dimorphism in kidney injury and repair
Abstract
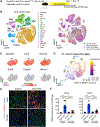
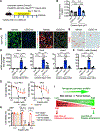
In both humans and mice, repair of acute kidney injury is worse in males than in females. Here, we provide evidence that this sexual dimorphism results from sex differences in ferroptosis, an iron-dependent, lipid-peroxidation-driven regulated cell death. Using genetic and single-cell transcriptomic approaches in mice, we report that female sex confers striking protection against ferroptosis, which was experimentally induced in proximal tubular (PT) cells by deleting glutathione peroxidase 4 (Gpx4). Single-cell transcriptomic analyses further identify the NFE2-related factor 2 (NRF2) antioxidant protective pathway as a female resilience mechanism against ferroptosis. Genetic inhibition and pharmacological activation studies show that NRF2 controls PT cell fate and plasticity by regulating ferroptosis. Importantly, pharmacological NRF2 activation protects male PT cells from ferroptosis and improves cellular plasticity as in females. Our data highlight NRF2 as a potential therapeutic target to prevent failed renal repair after acute kidney injury in both sexes by modulating cellular plasticity.
Keywords: CP: Molecular biology; GPX4; NRF2; acute kidney injury; cell fate; cellular plasticity; ferroptosis; glutathione Peroxidase 4; kidney injury and repair; sexual dimorphism; single-cell transcriptomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, and Rosenal T. (2005). Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit. Care 9, R700–R709. 10.1186/cc3879. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

