Large-scale all-optical dissection of motor cortex connectivity shows a segregated organization of mouse forelimb representations
- PMID: 36351410
- PMCID: PMC10073205
- DOI: 10.1016/j.celrep.2022.111627
Large-scale all-optical dissection of motor cortex connectivity shows a segregated organization of mouse forelimb representations
Abstract
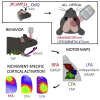
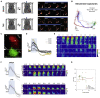
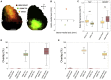
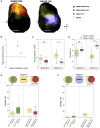
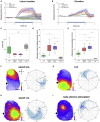
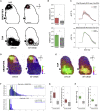
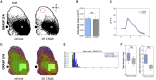
In rodent motor cortex, the rostral forelimb area (RFA) and the caudal forelimb area (CFA) are major actors in orchestrating the control of complex forelimb movements. However, their intrinsic connectivity and reciprocal functional organization are still unclear, limiting our understanding of how the brain coordinates and executes voluntary movements. Here, we causally probe cortical connectivity and activation patterns triggered by transcranial optogenetic stimulation of ethologically relevant complex movements exploiting a large-scale all-optical method in awake mice. Results show specific activation features for each movement class, providing evidence for a segregated functional organization of CFA and RFA. Importantly, we identify a second discrete lateral grasping representation area, namely the lateral forelimb area (LFA), with unique connectivity and activation patterns. Therefore, we propose the LFA as a distinct forelimb representation in the mouse somatotopic motor map.
Keywords: CFA; CP: Neuroscience; ChR2; RFA; calcium imaging; grasping; in vivo; jRCaMP1a; motor cortex organization; motor mapping; optogenetics; wide-field microscopy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
Distinct Functional Modules for Discrete and Rhythmic Forelimb Movements in the Mouse Motor Cortex.J Neurosci. 2015 Sep 30;35(39):13311-22. doi: 10.1523/JNEUROSCI.2731-15.2015. J Neurosci. 2015. PMID: 26424880 Free PMC article.
-
In vivo optogenetic tracing of functional corticocortical connections between motor forelimb areas.Front Neural Circuits. 2013 Apr 1;7:55. doi: 10.3389/fncir.2013.00055. eCollection 2013. Front Neural Circuits. 2013. PMID: 23554588 Free PMC article.
-
Ipsilateral-Dominant Control of Limb Movements in Rodent Posterior Parietal Cortex.J Neurosci. 2019 Jan 16;39(3):485-502. doi: 10.1523/JNEUROSCI.1584-18.2018. Epub 2018 Nov 26. J Neurosci. 2019. PMID: 30478035 Free PMC article.
-
Common marmoset as a model primate for study of the motor control system.Curr Opin Neurobiol. 2020 Oct;64:103-110. doi: 10.1016/j.conb.2020.02.013. Epub 2020 May 6. Curr Opin Neurobiol. 2020. PMID: 32389894 Review.
-
Mapping Structure-Function Relationships in the Brain.Biol Psychiatry Cogn Neurosci Neuroimaging. 2019 Jun;4(6):510-521. doi: 10.1016/j.bpsc.2018.10.005. Epub 2018 Oct 29. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. PMID: 30528965 Free PMC article. Review.
Cited by
-
Topographical and cell type-specific connectivity of rostral and caudal forelimb corticospinal neuron populations.Cell Rep. 2024 Apr 23;43(4):113993. doi: 10.1016/j.celrep.2024.113993. Epub 2024 Mar 27. Cell Rep. 2024. PMID: 38551963 Free PMC article.
-
Age-dependent cortical overconnectivity in Shank3 mice is reversed by anesthesia.Transl Psychiatry. 2025 Apr 19;15(1):154. doi: 10.1038/s41398-025-03377-5. Transl Psychiatry. 2025. PMID: 40253406 Free PMC article.
-
Group ICA of wide-field calcium imaging data reveals the retrosplenial cortex as a major contributor to cortical activity during anesthesia.Front Cell Neurosci. 2024 May 10;18:1258793. doi: 10.3389/fncel.2024.1258793. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38799987 Free PMC article.
-
Implantable silicon neural probes with nanophotonic phased arrays for single-lobe beam steering.Commun Eng. 2024 Dec 18;3(1):182. doi: 10.1038/s44172-024-00328-8. Commun Eng. 2024. PMID: 39695300 Free PMC article.
-
Updated Toolbox for Assessing Neuronal Network Reconstruction after Cell Therapy.Bioengineering (Basel). 2024 May 14;11(5):487. doi: 10.3390/bioengineering11050487. Bioengineering (Basel). 2024. PMID: 38790353 Free PMC article. Review.
References
-
- Akerboom J., Carreras Calderón N., Tian L., Wabnig S., Prigge M., Tolö J., Gordus A., Orger M.B., Severi K.E., Macklin J.J., Looger L.L. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. - DOI - PMC - PubMed
-
- Baker A., Kalmbach B., Morishima M., Kim J., Juavinett A., Li N., Dembrow N. Specialized subpopulations of deep-layer pyramidal neurons in the neocortex: bridging cellular properties to functional consequences. J. Neurosci. 2018;38:5441–5455. doi: 10.1523/JNEUROSCI.0150-18.2018. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

