CyclinD2-mediated regulation of neurogenic output from the retinal ciliary margin is perturbed in albinism
- PMID: 36351424
- PMCID: PMC9822872
- DOI: 10.1016/j.neuron.2022.10.025
CyclinD2-mediated regulation of neurogenic output from the retinal ciliary margin is perturbed in albinism
Abstract
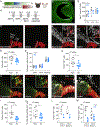
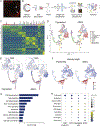
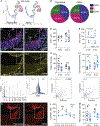
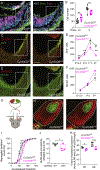
In albinism, aberrations in the ipsi-/contralateral retinal ganglion cell (RGC) ratio compromise the functional integrity of the binocular circuit. Here, we focus on the mouse ciliary margin zone (CMZ), a neurogenic niche at the embryonic peripheral retina, to investigate developmental processes regulating RGC neurogenesis and identity acquisition. We found that the mouse ventral CMZ generates predominantly ipsilaterally projecting RGCs, but this output is altered in the albino visual system because of CyclinD2 downregulation and disturbed timing of the cell cycle. Consequently, albino as well as CyclinD2-deficient pigmented mice exhibit diminished ipsilateral retinogeniculate projection and poor depth perception. In albino mice, pharmacological stimulation of calcium channels, known to upregulate CyclinD2 in other cell types, augmented CyclinD2-dependent neurogenesis of ipsilateral RGCs and improved stereopsis. Together, these results implicate CMZ neurogenesis and its regulators as critical for the formation and function of the mammalian binocular circuit.
Keywords: CyclinD2; RPE; albinism; binocular vision; ciliary margin; ipsilateral projection; neurogenesis; retinal ganglion cells; single-cell RNA seq.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

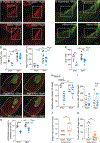
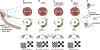
Comment in
-
Sticking to your side: A niche for the development of ipsilateral retinal projections.Neuron. 2023 Jan 4;111(1):5-8. doi: 10.1016/j.neuron.2022.12.017. Neuron. 2023. PMID: 36603550
References
-
- BALASUBRAMANIAN R, MIN X, QUINN PMJ, LO GIUDICE Q, TAO C, POLANCO K, MAKRIDES N, PEREGRIN J, BOUAZIZ M, MAO Y, WANG Q, DA COSTA BL, BUENAVENTURA D, WANG F, MA L, TSANG SH, FABRE PJ & ZHANG X 2021. Phase transition specified by a binary code patterns the vertebrate eye cup. Science Advances, 7. - PMC - PubMed
-
- BELANGER MC, ROBERT B & CAYOUETTE M 2017. Msx1-Positive Progenitors in the Retinal Ciliary Margin Give Rise to Both Neural and Non-neural Progenies in Mammals. Dev Cell, 40, 137–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

