EMILIN1 deficiency causes arterial tortuosity with osteopenia and connects impaired elastogenesis with defective collagen fibrillogenesis
- PMID: 36351433
- PMCID: PMC9748297
- DOI: 10.1016/j.ajhg.2022.10.010
EMILIN1 deficiency causes arterial tortuosity with osteopenia and connects impaired elastogenesis with defective collagen fibrillogenesis
Abstract
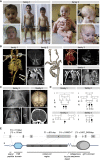
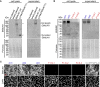
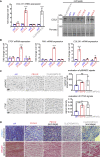
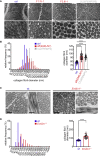
EMILIN1 (elastin-microfibril-interface-located-protein-1) is a structural component of the elastic fiber network and localizes to the interface between the fibrillin microfibril scaffold and the elastin core. How EMILIN1 contributes to connective tissue integrity is not fully understood. Here, we report bi-allelic EMILIN1 loss-of-function variants causative for an entity combining cutis laxa, arterial tortuosity, aneurysm formation, and bone fragility, resembling autosomal-recessive cutis laxa type 1B, due to EFEMP2 (FBLN4) deficiency. In both humans and mice, absence of EMILIN1 impairs EFEMP2 extracellular matrix deposition and LOX activity resulting in impaired elastogenesis, reduced collagen crosslinking, and aberrant growth factor signaling. Collagen fiber ultrastructure and histopathology in EMILIN1- or EFEMP2-deficient skin and aorta corroborate these findings and murine Emilin1-/- femora show abnormal trabecular bone formation and strength. Altogether, EMILIN1 connects elastic fiber network with collagen fibril formation, relevant for both bone and vascular tissue homeostasis.
Keywords: EFEMP2; EMILIN1; LOX; aortic aneurysm; arterial tortuosity; collagen; cutis laxa; elastic fiber; extracellular matrix; fracture.
Copyright © 2022 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Schmelzer C.E.H., Hedtke T., Heinz A. Unique molecular networks: Formation and role of elastin cross-links. IUBMB Life. 2020;72:842–854. - PubMed
-
- Schiavinato A., Keene D.R., Wohl A.P., Corallo D., Colombatti A., Wagener R., Paulsson M., Bonaldo P., Sengle G. Targeting of EMILIN-1 and EMILIN-2 to Fibrillin Microfibrils Facilitates their Incorporation into the Extracellular Matrix. J. Invest. Dermatol. 2016;136:1150–1160. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

