Interleukin-6 initiates muscle- and adipose tissue wasting in a novel C57BL/6 model of cancer-associated cachexia
- PMID: 36351437
- PMCID: PMC9891934
- DOI: 10.1002/jcsm.13109
Interleukin-6 initiates muscle- and adipose tissue wasting in a novel C57BL/6 model of cancer-associated cachexia
Abstract
Background: Cancer-associated cachexia (CAC) is a wasting syndrome drastically reducing efficacy of chemotherapy and life expectancy of patients. CAC affects up to 80% of cancer patients, yet the mechanisms underlying the disease are not well understood and no approved disease-specific medication exists. As a multiorgan disorder, CAC can only be studied on an organismal level. To cover the diverse aetiologies of CAC, researchers rely on the availability of a multifaceted pool of cancer models with varying degrees of cachexia symptoms. So far, no tumour model syngeneic to C57BL/6 mice exists that allows direct comparison between cachexigenic- and non-cachexigenic tumours.
Methods: MCA207 and CHX207 fibrosarcoma cells were intramuscularly implanted into male or female, 10-11-week-old C57BL/6J mice. Tumour tissues were subjected to magnetic resonance imaging, immunohistochemical-, and transcriptomic analysis. Mice were analysed for tumour growth, body weight and -composition, food- and water intake, locomotor activity, O2 consumption, CO2 production, circulating blood cells, metabolites, and tumourkines. Mice were sacrificed with same tumour weights in all groups. Adipose tissues were examined using high-resolution respirometry, lipolysis measurements in vitro and ex vivo, and radioactive tracer studies in vivo. Gene expression was determined in adipose- and muscle tissues by quantitative PCR and Western blotting analyses. Muscles and cultured myotubes were analysed histologically and by immunofluorescence microscopy for myofibre cross sectional area and myofibre diameter, respectively. Interleukin-6 (Il-6) was deleted from cancer cells using CRISPR/Cas9 mediated gene editing.
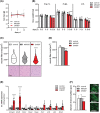
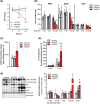
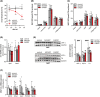
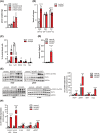
Results: CHX207, but not MCA207-tumour-bearing mice exhibited major clinical features of CAC, including systemic inflammation, increased plasma IL-6 concentrations (190 pg/mL, P ≤ 0.0001), increased energy expenditure (+28%, P ≤ 0.01), adipose tissue loss (-47%, P ≤ 0.0001), skeletal muscle wasting (-18%, P ≤ 0.001), and body weight reduction (-13%, P ≤ 0.01) 13 days after cancer cell inoculation. Adipose tissue loss resulted from reduced lipid uptake and -synthesis combined with increased lipolysis but was not associated with elevated beta-adrenergic signalling or adipose tissue browning. Muscle atrophy was evident by reduced myofibre cross sectional area (-21.8%, P ≤ 0.001), increased catabolic- and reduced anabolic signalling. Deletion of IL-6 from CHX207 cancer cells completely protected CHX207IL6KO -tumour-bearing mice from CAC.
Conclusions: In this study, we present CHX207 fibrosarcoma cells as a novel tool to investigate the mediators and metabolic consequences of CAC in C57BL/6 mice in comparison to non-cachectic MCA207-tumour-bearing mice. IL-6 represents an essential trigger for CAC development in CHX207-tumour-bearing mice.
Keywords: Adipose tissue; C57BL/6; Cachexia; Cancer; Interleukin-6.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors of this manuscript declare that they have no conflicts of interest.
Figures


References
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
-
- Tisdale MJ. Adipose Tissue Cachexia. In Adipose Tissue in Health and Disease. Weinheim, Germany: Wiley‐VCH Verlag GmbH & Co. KGaA; 2010. p 333–345.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

