Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir
- PMID: 36351451
- PMCID: PMC9849135
- DOI: 10.1038/s41586-022-05514-2
Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir
Abstract
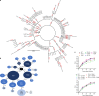
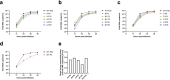
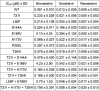
Nirmatrelvir, an oral antiviral targeting the 3CL protease of SARS-CoV-2, has been demonstrated to be clinically useful against COVID-19 (refs. 1,2). However, because SARS-CoV-2 has evolved to become resistant to other therapeutic modalities3-9, there is a concern that the same could occur for nirmatrelvir. Here we examined this possibility by in vitro passaging of SARS-CoV-2 in nirmatrelvir using two independent approaches, including one on a large scale. Indeed, highly resistant viruses emerged from both and their sequences showed a multitude of 3CL protease mutations. In the experiment peformed with many replicates, 53 independent viral lineages were selected with mutations observed at 23 different residues of the enzyme. Nevertheless, several common mutational pathways to nirmatrelvir resistance were preferred, with a majority of the viruses descending from T21I, P252L or T304I as precursor mutations. Construction and analysis of 13 recombinant SARS-CoV-2 clones showed that these mutations mediated only low-level resistance, whereas greater resistance required accumulation of additional mutations. E166V mutation conferred the strongest resistance (around 100-fold), but this mutation resulted in a loss of viral replicative fitness that was restored by compensatory changes such as L50F and T21I. Our findings indicate that SARS-CoV-2 resistance to nirmatrelvir does readily arise via multiple pathways in vitro, and the specific mutations observed herein form a strong foundation from which to study the mechanism of resistance in detail and to inform the design of next-generation protease inhibitors.
© 2022. The Author(s).
Conflict of interest statement
S.I., A.C. and D.D.H. are inventors on patent applications related to the development of inhibitors against the SARS-CoV-2 3CL protease. D.D.H. is a cofounder of TaiMed Biologics and RenBio, consultant to WuXi Biologics and Brii Biosciences and board director for Vicarious Surgical.
Figures




Update of
-
Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir.bioRxiv [Preprint]. 2022 Aug 18:2022.08.07.499047. doi: 10.1101/2022.08.07.499047. bioRxiv. 2022. Update in: Nature. 2023 Jan;613(7944):558-564. doi: 10.1038/s41586-022-05514-2. PMID: 36032976 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

