Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials
- PMID: 36351458
- PMCID: PMC7613836
- DOI: 10.1016/S0140-6736(22)02074-8
Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials
Abstract
Background: Large trials have shown that sodium glucose co-transporter-2 (SGLT2) inhibitors reduce the risk of adverse kidney and cardiovascular outcomes in patients with heart failure or chronic kidney disease, or with type 2 diabetes and high risk of atherosclerotic cardiovascular disease. None of the trials recruiting patients with and without diabetes were designed to assess outcomes separately in patients without diabetes.
Methods: We did a systematic review and meta-analysis of SGLT2 inhibitor trials. We searched the MEDLINE and Embase databases for trials published from database inception to Sept 5, 2022. SGLT2 inhibitor trials that were double-blind, placebo-controlled, performed in adults (age ≥18 years), large (≥500 participants per group), and at least 6 months in duration were included. Summary-level data used for analysis were extracted from published reports or provided by trial investigators, and inverse-variance-weighted meta-analyses were conducted to estimate treatment effects. The main efficacy outcomes were kidney disease progression (standardised to a definition of a sustained ≥50% decrease in estimated glomerular filtration rate [eGFR] from randomisation, a sustained low eGFR, end-stage kidney disease, or death from kidney failure), acute kidney injury, and a composite of cardiovascular death or hospitalisation for heart failure. Other outcomes were death from cardiovascular and non-cardiovascular disease considered separately, and the main safety outcomes were ketoacidosis and lower limb amputation. This study is registered with PROSPERO, CRD42022351618.
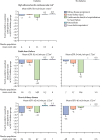
Findings: We identified 13 trials involving 90 413 participants. After exclusion of four participants with uncertain diabetes status, we analysed 90 409 participants (74 804 [82·7%] participants with diabetes [>99% with type 2 diabetes] and 15 605 [17·3%] without diabetes; trial-level mean baseline eGFR range 37-85 mL/min per 1·73 m2). Compared with placebo, allocation to an SGLT2 inhibitor reduced the risk of kidney disease progression by 37% (relative risk [RR] 0·63, 95% CI 0·58-0·69) with similar RRs in patients with and without diabetes. In the four chronic kidney disease trials, RRs were similar irrespective of primary kidney diagnosis. SGLT2 inhibitors reduced the risk of acute kidney injury by 23% (0·77, 0·70-0·84) and the risk of cardiovascular death or hospitalisation for heart failure by 23% (0·77, 0·74-0·81), again with similar effects in those with and without diabetes. SGLT2 inhibitors also reduced the risk of cardiovascular death (0·86, 0·81-0·92) but did not significantly reduce the risk of non-cardiovascular death (0·94, 0·88-1·02). For these mortality outcomes, RRs were similar in patients with and without diabetes. For all outcomes, results were broadly similar irrespective of trial mean baseline eGFR. Based on estimates of absolute effects, the absolute benefits of SGLT2 inhibition outweighed any serious hazards of ketoacidosis or amputation.
Interpretation: In addition to the established cardiovascular benefits of SGLT2 inhibitors, the randomised data support their use for modifying risk of kidney disease progression and acute kidney injury, not only in patients with type 2 diabetes at high cardiovascular risk, but also in patients with chronic kidney disease or heart failure irrespective of diabetes status, primary kidney disease, or kidney function.
Funding: UK Medical Research Council and Kidney Research UK.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NS, RH, KJM, AJR, SYAN, DZ, PJ, DP, MJL, CB, JRE, and WGH report institutional grant funding from Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial. NS additionally reports institutional grant funding from Novo Nordisk. RH additionally reports institutional grant funding from Novartis; and trial drug supply from Roche and Regeneron. CB additionally reports grant funding from the UK Medical Research Council, the UK National Institute for Health and Care Research Health Technology Assessment, and Health Data Research UK; and advisory roles for Merck, the National Institute for Health and Care Research Health Technology Assessment, the British Heart Foundation, and the European Society of Cardiology. WGH additionally reports funding from the UK Medical Research Council–Kidney Research UK Professor David Kerr Clinician Scientist Award. BLN reports consultancy fees and honorarium paid to his institution by AstraZeneca, Bayer, Boehringer Ingelheim, Cambridge Healthcare Research, American Diabetes Association, Renal Society of Australasia and Janssen; and advisory board membership (fees paid to institution) with AstraZeneca, Bayer, and Boehringer Ingelheim. SJH and MB are full-time employees of Boehringer Ingelheim International. SDA reports institutional grant funding from Vifor Int and Abbott Vascular; consultancy or advisory board fees from CVRx, Amgen, Respicardia, Novo Nordisk, Brahms, Novartis, Sanofi, and Cordio; and additional leadership or advisory board roles with Vifor Int, Bayer, Boehringer Ingelheim, Servier, Abbott Vascular, Impulse Dynamics, AstraZeneca, Bioventrix, Janssen, Cardior, V-Wave, Cardiac Dimensions, and Occlutech. JB reports consultancy fees and honorarium from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sequana Medical, and Vifor. DZIC reports institutional grant funding from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, CSL-Behring, and Novo Nordisk; and consultancy fees and honorarium from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, Bristol Myers Squibb, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene, Lexicon, and Novo Nordisk. JBG reports institutional grant funding from Boehringer Ingelheim-Lilly, Merck, Roche, and Sanofi and Lexicon; and consultancy fees from Boehringer Ingelheim-Lilly, Bayer, AstraZeneca, Sanofi and Lexicon, Hawthorne Effect and Omada, Pfizer, Valo, Anji, Vertex, and Novo Nordisk. C-CL is an employee of Merck Sharp & Dohme (a subsidiary of Merck & Co) and owns stock and/or stock options in Merck & Co. FRMcC reports grant funding from NIDDK, Satellite Healthcare, Advanced Medical, and Fifth Eye; and consultancy fees from GlaxoSmithKline, Advanced Medical, and Zydus Therapeutics. DKMcG reports consultancy fees from Merck & Co, Applied Therapeutics, Metavant, Sanofi, Afimmune, Lilly USA, Boehringer Ingelheim, Novo Nordisk, Bayer, GlaxoSmithKline, Lexicon, Altimmune, and Esperion; and other honorarium from Kirkland & Ellis, Pfizer, GlaxoSmithKline, Janssen, Afimmune, Sanofi, Boehringer Ingelheim, Merck & Co, AstraZeneca, Novo Nordisk, Esperion, and Lilly USA. JJVMcM reports institutional grant funding from AstraZeneca; consultancy fees from Abbott, Alkem Metabolics, Eris Lifesciences, Hikma, Lupin, Sun Pharmaceuticals, Heart.Org (Medscape Cardiology), ProAdWise Communications, Radcliffe Cardiology, Servier, and The Corpus; and fees paid to his institution for other advisory roles by Cytokinetics, Amgen, AstraZeneca, Theracos, Ionis Pharmaceuticals, DalCor, Cardurion, Novartis, GlaxoSmithKline, Bayer, KBP Biosciences, Boehringer Ingelheim, and Bristol Myers Squibb. MP reports personal fees from AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, and Salamandra. VP reports consultancy fees, honorarium, or advisory roles supported by AbbVie, Bayer, Boehringer Ingelheim, Chinook, GlaxoSmithKline, Janssen, Pfizer, AstraZeneca, Baxter, Eli Lilly, Gilead, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Retrophin, Roche, Sanofi, Servier, and Vitae. MSS reports institutional grant funding from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, Intarcia, Ionis, Medicines Company, MedImmune, Merck, Novartis, Pfizer, and Quark Pharmaceuticals; and consultancy fees from Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol Myers Squibb, and DalCor. SDS reports institutional grant funding from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, the National Heart, Lung, and Blood Institute (US National Institutes of Health), Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and consultancy fees from Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Puretech Health. MV reports grant funding or advisory board fees from Amgen, AstraZeneca, American Regent, Baxter HealthCare, Bayer, Boehringer Ingelheim, Cytokinetics, Pharmacosmos, Relypsa, Novartis, Roche Diagnostics, Lexicon Pharmaceuticals, Galmed, Occlutech, Impulse Dynamics, Sanofi, and Tricog Health; speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics; and actively participates on clinical trial committees for studies sponsored by Galmed, Novartis, Bayer, Occlutech, and Impulse Dynamics. CW reports institutional grant funding from Boehringer Ingelheim; and consultancy fees and honorarium from Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, and Bayer. SDW reports institutional grant funding from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, Janssen Research and Development, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, Softcell Medical, The Medicines Company, and Zora Biosciences; and consultancy fees from AstraZeneca, Boston Clinical Research Institute, Icon Clinical, and Novo Nordisk. FZ reports consultancy fees from Amgen, Applied therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cardior, Cereno Scientific, CEVA, Cellprothera, CVRx, Novartis, Novo Nordisk, Servier, Merck, Bristol Myers Squibb; and honorarium or other personal fees from Boehringer Ingelheim, Merck, Bayer, Vifor, Fresenius, Roche Diagnostics, Hogan and Lovells, and Acceleron. HJLH reports grant funding from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk; consultancy fees from AstraZeneca, AbbVie, Bayer, Boehringer Ingelheim, CSL Behring, Chinook, Dimerix, Eli Lilly, Gilead, Goldfinch Bio, Merck, Novartis, Novo Nordisk, Janssen, and Travere Therapeutics; and other payment or honorarium from AstraZeneca, Novo Nordisk, and Eli Lilly. MJL additionally reports institutional grant funding from Novartis and Janssen; and trial drug supply from Roche and Regeneron.
Figures




Comment in
-
Implementation, not hesitation, for SGLT2 inhibition as foundational therapy for chronic kidney disease.Lancet. 2022 Nov 19;400(10365):1745-1747. doi: 10.1016/S0140-6736(22)02164-X. Epub 2022 Nov 6. Lancet. 2022. PMID: 36351457 No abstract available.
-
Kidney benefits of SLGT2 inhibitors: evidence from clinical trials.Nat Rev Nephrol. 2023 Jan;19(1):3. doi: 10.1038/s41581-022-00659-9. Nat Rev Nephrol. 2023. PMID: 36450917 No abstract available.
-
SGLT2 inhibitors reduce adverse renal and CV outcomes in patients with or without diabetes.Ann Intern Med. 2023 Mar;176(3):JC27. doi: 10.7326/J23-0002. Epub 2023 Mar 7. Ann Intern Med. 2023. PMID: 36877973
References
-
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. - PubMed
-
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. - PubMed
-
- Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. - PubMed
-
- Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

