The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case
- PMID: 36351479
- PMCID: PMC9637289
- DOI: 10.1016/j.xphs.2022.11.001
The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case
Abstract
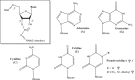
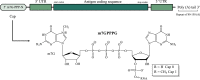
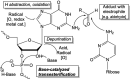
The remarkable impact of mRNA vaccines on mitigating disease and improving public health has been amply demonstrated during the COVID-19 pandemic. Many new mRNA-based vaccine and therapeutic candidates are in development, yet the current reality of their stability limitations requires their frozen storage. Numerous challenges remain to improve formulated mRNA stability and enable refrigerator storage, and this review provides an update on developments to tackle this multi-faceted stability challenge. We describe the chemistry underlying mRNA degradation during storage and highlight how lipid nanoparticle (LNP) formulations are a double-edged sword: while LNPs protect mRNA against enzymatic degradation, interactions with and between LNP excipients introduce additional risks for mRNA degradation. We also discuss strategies to improve mRNA stability both as a drug substance (DS) and a drug product (DP) including the (1) design of the mRNA molecule (nucleotide selection, primary and secondary structures), (2) physical state of the mRNA-LNP complexes, (3) formulation composition and purity of the components, and (4) DS and DP manufacturing processes. Finally, we summarize analytical control strategies to monitor and assure the stability of mRNA-based candidates, and advocate for an integrated analytical and formulation development approach to further improve their storage, transport, and in-use stability profiles.
Keywords: Degradation mechanisms; Formulation; Impurities; Ionizable lipid; LNP; Lipid nanoparticles; Physical and chemical analysis; Shelf life; Stability; Vaccine; mRNA structure.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. Published 2022. Accessed October 2, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- EMA. Adapted vaccine targeting BA.4 and BA.5 Omicron variants and original SARS-CoV-2 recommended for approval. Published 2022. Accessed October 2, 2022. https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omic...
-
- Barbier AJ, Yujie Jiang A, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. doi:10.1038/s41587-022-01294-2 - PubMed
-
- Garber K. mRNA pioneers refocus on therapeutics. Nat Rev Drug Discov. 2022;21:699–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

