Synergistic Protection against Secondary Pneumococcal Infection by Human Monoclonal Antibodies Targeting Distinct Epitopes
- PMID: 36351696
- PMCID: PMC9898123
- DOI: 10.4049/jimmunol.2200349
Synergistic Protection against Secondary Pneumococcal Infection by Human Monoclonal Antibodies Targeting Distinct Epitopes
Abstract
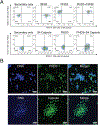
Streptococcus pneumoniae persists as a leading cause of bacterial pneumonia despite the widespread use of polysaccharide-based vaccines. The limited serotype coverage of current vaccines has led to increased incidence of nonvaccine serotypes, as well as an increase in antibiotic resistance among these serotypes. Pneumococcal infection often follows a primary viral infection such as influenza virus, which hinders host defense and results in bacterial spread to the lungs. We previously isolated human monoclonal Abs (mAbs) against the conserved surface Ag pneumococcal histidine triad protein D (PhtD), and we demonstrated that mAbs to this Ag are protective against lethal pneumococcal challenge prophylactically and therapeutically. In this study, we elucidated the mechanism of protection of a protective anti-pneumococcal human mAb, PhtD3, which is mediated by the presence of complement and macrophages in a mouse model of pneumococcal infection. Treatment with mAb PhtD3 reduced blood and lung bacterial burden in mice, and mAb PhtD3 is able to bind to bacteria in the presence of the capsular polysaccharide, indicating exposure of surface PhtD on encapsulated bacteria. In a mouse model of secondary pneumococcal infection, protection mediated by mAb PhtD3 and another mAb targeting a different epitope, PhtD7, was reduced; however, robust protection was restored by combining mAb PhtD3 with mAb PhtD7, indicating a synergistic effect. Overall, these studies provide new insights into anti-pneumococcal mAb protection and demonstrate, to our knowledge, for the first time, that mAbs to pneumococcal surface proteins can protect against secondary pneumococcal infection in the mouse model.
Copyright © 2022 by The American Association of Immunologists, Inc.
Figures
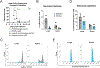

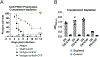
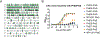
Similar articles
-
Human monoclonal antibodies protect against viral-mediated pneumococcal superinfection.Front Immunol. 2024 Jun 12;15:1364622. doi: 10.3389/fimmu.2024.1364622. eCollection 2024. Front Immunol. 2024. PMID: 38933273 Free PMC article.
-
Broadly Reactive Human Monoclonal Antibodies Targeting the Pneumococcal Histidine Triad Protein Protect against Fatal Pneumococcal Infection.Infect Immun. 2021 Apr 16;89(5):e00747-20. doi: 10.1128/IAI.00747-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33649050 Free PMC article.
-
Antibodies to PcpA and PhtD protect mice against Streptococcus pneumoniae by a macrophage- and complement-dependent mechanism.Hum Vaccin Immunother. 2018 Feb 1;14(2):489-494. doi: 10.1080/21645515.2017.1403698. Epub 2017 Dec 14. Hum Vaccin Immunother. 2018. PMID: 29135332 Free PMC article.
-
Diverse Mechanisms of Protective Anti-Pneumococcal Antibodies.Front Cell Infect Microbiol. 2022 Jan 28;12:824788. doi: 10.3389/fcimb.2022.824788. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35155281 Free PMC article. Review.
-
A Jack of All Trades: The Role of Pneumococcal Surface Protein A in the Pathogenesis of Streptococcus pneumoniae.Front Cell Infect Microbiol. 2022 Feb 2;12:826264. doi: 10.3389/fcimb.2022.826264. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186799 Free PMC article. Review.
Cited by
-
Promoting Fc-Fc interactions between anti-capsular antibodies provides strong immune protection against Streptococcus pneumoniae.Elife. 2023 Mar 22;12:e80669. doi: 10.7554/eLife.80669. Elife. 2023. PMID: 36947116 Free PMC article.
-
Bacterium-like Particles from Corynebacterium pseudodiphtheriticum as Mucosal Adjuvant for the Development of Pneumococcal Vaccines.Vaccines (Basel). 2024 Apr 12;12(4):412. doi: 10.3390/vaccines12040412. Vaccines (Basel). 2024. PMID: 38675794 Free PMC article.
-
Antibodies as drugs-a Keystone Symposia report.Ann N Y Acad Sci. 2023 Jan;1519(1):153-166. doi: 10.1111/nyas.14915. Epub 2022 Nov 16. Ann N Y Acad Sci. 2023. PMID: 36382536 Free PMC article.
-
Human monoclonal antibodies protect against viral-mediated pneumococcal superinfection.Front Immunol. 2024 Jun 12;15:1364622. doi: 10.3389/fimmu.2024.1364622. eCollection 2024. Front Immunol. 2024. PMID: 38933273 Free PMC article.
-
Outsmarting Pathogens with Antibody Engineering.Annu Rev Chem Biomol Eng. 2023 Jun 8;14:217-241. doi: 10.1146/annurev-chembioeng-101121-084508. Epub 2023 Mar 14. Annu Rev Chem Biomol Eng. 2023. PMID: 36917814 Free PMC article. Review.
References
-
- WHO. 2017. Who Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed,. GENEVA.
-
- Bonnave C, Mertens D, Peetermans W, Cobbaert K, Ghesquiere B, Deschodt M, and Flamaing J. 2019. Adult vaccination for pneumococcal disease: a comparison of the national guidelines in Europe. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol 38: 785–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

