Efficacy of neuromuscular electrical stimulation with combined low and high frequencies on body composition, peripheral muscle function and exercise tolerance in patients with chronic kidney disease undergoing haemodialysis: a protocol for a randomised, double-blind clinical trial
- PMID: 36351736
- PMCID: PMC9664278
- DOI: 10.1136/bmjopen-2022-062062
Efficacy of neuromuscular electrical stimulation with combined low and high frequencies on body composition, peripheral muscle function and exercise tolerance in patients with chronic kidney disease undergoing haemodialysis: a protocol for a randomised, double-blind clinical trial
Abstract
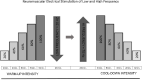
Introduction: Neuromuscular electrical stimulation (NMES) as an adjunctive strategy to increase isolated muscular strength or endurance has been widely investigated in patients with chronic kidney disease (CKD) undergoing haemodialysis (HD). However, the efficacy of combined low and high frequencies, to improve both muscular strength and endurance, is unknown. This trial aims to evaluate the efficacy of this combined NMES strategy in this population.
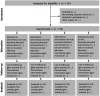
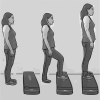
Methods and analysis: This is a randomised controlled trial with blinded assessments and analysis. A total of 56 patients with CKD undergoing HD will be recruited and randomised to an NMES protocol. The evaluations will be performed on three different days at baseline and after 24 sessions of follow-up. Assessments will include the background, insulin-like growth factor, lactate measurement, malnutrition and inflammation score evaluation, an electrical bioimpedance examination, global muscular evaluation by means of the Medical Research Council scale, handgrip strength evaluation, muscular isokinetic evaluation of lower limbs, 6 min step test performance and quality of life (QoL) questionnaire with emphasis on physical function. The patients will be allocated in one of the following four groups: 1) combined low and high frequencies; 2) low frequency; 3) high frequency; and 4) sham stimulation with minimal intensity to generate only sensory perception (with no visible contraction). In all groups, the intensity throughout the session will be the highest tolerated by patient (except for control group). The primary endpoint is the change of peripheral muscle function (muscular strength and endurance). The secondary endpoints will be the changes of body composition; muscle trophism; exercise tolerance; QoL; and nutritional, inflammatory, and metabolic markers. The findings of this study are expected to provide valuable knowledge on how to optimise the NMES intervention, with improvements in both muscle strength and endurance.
Ethics and dissemination: This protocol has been approved by the Ethics Committee on Research with Humans of Hospital Sírio-Libanês (approval no. 24337707). Written informed consent will be obtained from each participant. The results of the study will be published in peer-reviewed journals.
Trial registration number: NCT03779126.
Keywords: Chronic renal failure; Dialysis; Nephrology; REHABILITATION MEDICINE.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Effects of Neuromuscular Electrical Stimulation During Hemodialysis on Peripheral Muscle Strength and Exercise Capacity: A Randomized Clinical Trial.Arch Phys Med Rehabil. 2017 May;98(5):822-831.e1. doi: 10.1016/j.apmr.2016.12.009. Epub 2017 Jan 16. Arch Phys Med Rehabil. 2017. PMID: 28093194 Clinical Trial.
-
Single-centre, double-blinded, randomised placebo-controlled trial to determine the effect of a 12-week home-based programme of footplate neuromuscular electrical stimulation on walking capacity in people with peripheral artery disease: a protocol for the Foot-PAD trial.BMJ Open. 2025 Jan 25;15(1):e093162. doi: 10.1136/bmjopen-2024-093162. BMJ Open. 2025. PMID: 39863411 Free PMC article.
-
Comparison of intradialytic neuromuscular electrical stimulation and oral nutritional supplements in hemodialysis patients: study protocol for a multicenter, parallel-group, randomized controlled trial in Korea.Trials. 2021 Dec 20;22(1):942. doi: 10.1186/s13063-021-05918-x. Trials. 2021. PMID: 34930408 Free PMC article.
-
Intradialytic neuromuscular electrical stimulation improves functional capacity and muscle strength in people receiving haemodialysis: a systematic review.J Physiother. 2020 Apr;66(2):89-96. doi: 10.1016/j.jphys.2020.03.006. Epub 2020 Apr 11. J Physiother. 2020. PMID: 32291224
-
Effectiveness of neuromuscular electrical stimulation for the rehabilitation of moderate-to-severe COPD: a meta-analysis.Int J Chron Obstruct Pulmon Dis. 2016 Nov 28;11:2965-2975. doi: 10.2147/COPD.S120555. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27932876 Free PMC article. Review.
Cited by
-
Prevalence of locomotive syndrome and associated factors in patients receiving hemodialysis.Medicine (Baltimore). 2025 Jan 3;104(1):e40007. doi: 10.1097/MD.0000000000040007. Medicine (Baltimore). 2025. PMID: 40184099 Free PMC article.
-
The phase angle cut-off point capable of discriminating hemodialysis patients with reduced exercise tolerance: a cross-sectional study.BMC Sports Sci Med Rehabil. 2024 Feb 2;16(1):34. doi: 10.1186/s13102-024-00825-5. BMC Sports Sci Med Rehabil. 2024. PMID: 38308310 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
