The Role of Platelet-Derived Growth Factor in Focal Segmental Glomerulosclerosis
- PMID: 36351762
- PMCID: PMC10103089
- DOI: 10.1681/ASN.2022040491
The Role of Platelet-Derived Growth Factor in Focal Segmental Glomerulosclerosis
Abstract
Background: FSGS is the final common pathway to nephron loss in most forms of severe or progressive glomerular injury. Although podocyte injury initiates FSGS, parietal epithelial cells (PECs) are the main effectors. Because PDGF takes part in fibrotic processes, we hypothesized that the ligand PDGF-B and its receptor PDGFR- β participate in the origin and progression of FSGS.
Methods: We challenged Thy1.1 transgenic mice, which express Thy1.1 in the podocytes, with anti-Thy1.1 antibody to study the progression of FSGS. We investigated the role of PDGF in FSGS using challenged Thy1.1 mice, 5/6 nephrectomized mice, Col4 -/- (Alport) mice, patient kidney biopsies, and primary murine PECs, and challenged Thy1.1 mice treated with neutralizing anti-PDGF-B antibody therapy.
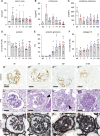
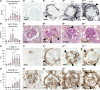
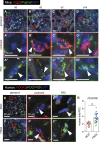
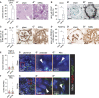
Results: The unchallenged Thy1.1 mice developed only mild spontaneous FSGS, whereas challenged mice developed progressive FSGS accompanied by a decline in kidney function. PEC activation, proliferation, and profibrotic phenotypic switch drove the FSGS. During disease, PDGF-B was upregulated in podocytes, whereas PDGFR- β was upregulated in PECs from both mice and patients with FSGS. Short- and long-term treatment with PDGF-B neutralizing antibody improved kidney function and reduced FSGS, PEC proliferation, and profibrotic activation. In vitro , stimulation of primary murine PECs with PDGF-B recapitulated in vivo findings with PEC activation and proliferation, which was inhibited by PDGF-B antibody or imatinib.
Conclusion: PDGF-B-PDGFR- β molecular crosstalk between podocytes and PECs drives glomerulosclerosis and the progression of FSGS.
Podcast: This article contains a podcast at.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
P. Boor reports consultancy fees from Bristol Myers Squibb. J. Floege reports consultancy fees and honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Calliditas, Chinook, Novo Nordisk, Novartis, Omeros, Travere, Vifor, and Visterra; reports advisory or leadership role with Calliditas, Omeros, and Travere; and is on the speakers bureau for Amgen, AstraZeneca, Novartis, and Vifor. All remaining authors have nothing to disclose.
Figures




References
-
- Nagata M, Horita S, Shu Y, Shibata S, Hattori M, Ito K, et al. : Phenotypic characteristics and cyclin-dependent kinase inhibitors repression in hyperplastic epithelial pathology in idiopathic focal segmental glomerulosclerosis. Lab Invest 80: 869–880, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

