Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer
- PMID: 36351793
- PMCID: PMC10037540
- DOI: 10.1136/heartjnl-2022-321545
Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer
Abstract
Objectives: Sodium-glucose cotransporter-2 inhibitors (SGLT2i) reduce heart failure (HF) in at-risk patients and may possess antitumour effects. We examined the effect of SGLT2i on HF and mortality among patients with cancer and diabetes.
Methods: This was a retrospective propensity score-matched cohort study involving adult patients with type 2 diabetes mellitus diagnosed with cancer between January 2010 and December 2021. The primary outcomes were hospitalisation for incident HF and all-cause mortality. The secondary outcomes were serious adverse events associated with SGLT2i.
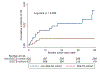
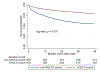
Results: From a total of 8640 patients, 878 SGLT2i recipients were matched to non-recipients. During a median follow-up of 18.8 months, SGLT2i recipients had a threefold lower rate of hospitalisation for incident HF compared with non-SGLT2i recipients (2.92 vs 8.95 per 1000 patient-years, p=0.018). In Cox regression and competing regression models, SGLT2i were associated with a 72% reduction in the risk of hospitalisation for HF (HR 0.28 (95% CI: 0.11 to 0.77), p=0.013; subdistribution HR 0.32 (95% CI: 0.12 to 0.84), p=0.021). The use of SGLT2i was also associated with a higher overall survival (85.3% vs 63.0% at 2 years, p<0.001). The risk of serious adverse events such as hypoglycaemia and sepsis was similar between the two groups.
Conclusions: The use of SGLT2i was associated with a lower rate of incident HF and prolonged overall survival in patients with cancer with diabetes mellitus.
Keywords: Heart failure.
© Author(s) (or their employer(s)) 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TGN has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, BMS, H3-Biomedicine, AbbVie, C4-Therapeutics, Roche, Sanofi and Genentech, outside of the current work. TGN has received grant funding from AstraZeneca and BMS. PA has served as a consultant for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, Astra Zeneca, Genentech, Xencor, outside of the current work. PA has received research funding from Kite, Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM, and speaking honoraria from Merck and BMS, all unrelated to the current work. LRP has stock in Johnson and Johnson, Exact Sciences, Adverum Biotechnology, Crisper Therapeutics, Editas Med., Integra Lifesciences, Medtronic, Seagen Inc., Shockwave Medical, outside of the current work. All other authors have no conflict of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
