Phenobarbital in Nuclear Receptor Activation: An Update
- PMID: 36351837
- PMCID: PMC9900862
- DOI: 10.1124/dmd.122.000859
Phenobarbital in Nuclear Receptor Activation: An Update
Abstract
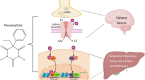
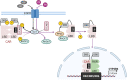
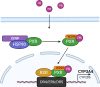
Phenobarbital (PB) is a commonly prescribed anti-epileptic drug that can also benefit newborns from hyperbilirubinemia. Being the first drug demonstrating hepatic induction of cytochrome P450 (CYP), PB has since been broadly used as a model compound to study xenobiotic-induced drug metabolism and clearance. Mechanistically, PB-mediated CYP induction is linked to a number of nuclear receptors, such as the constitutive androstane receptor (CAR), pregnane X receptor (PXR), and estrogen receptor α, with CAR being the predominant regulator. Unlike prototypical agonistic ligands, PB-mediated activation of CAR does not involve direct binding with the receptor. Instead, dephosphorylation of threonine 38 in the DNA-binding domain of CAR was delineated as a key signaling event underlying PB-mediated indirect activation of CAR. Further studies revealed that such phosphorylation sites appear to be highly conserved among most human nuclear receptors. Interestingly, while PB is a pan-CAR activator in both animals and humans, PB activates human but not mouse PXR. The species-specific role of PB in gene regulation is a key determinant of its implication in xenobiotic metabolism, drug-drug interactions, energy homeostasis, and cell proliferation. In this review, we summarize the recent progress in our understanding of PB-provoked transactivation of nuclear receptors with a focus on CAR and PXR. SIGNIFICANCE STATEMENT: Extensive studies using PB as a research tool have significantly advanced our understanding of the molecular basis underlying nuclear receptor-mediated drug metabolism, drug-drug interactions, energy homeostasis, and cell proliferation. In particular, CAR has been established as a cell signaling-regulated nuclear receptor in addition to ligand-dependent functionality. This mini-review highlights the mechanisms by which PB transactivates CAR and PXR.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Nuclear receptor phosphorylation in xenobiotic signal transduction.J Biol Chem. 2020 Nov 6;295(45):15210-15225. doi: 10.1074/jbc.REV120.007933. Epub 2020 Aug 11. J Biol Chem. 2020. PMID: 32788213 Free PMC article. Review.
-
Phenobarbital induces cell cycle transcriptional responses in mouse liver humanized for constitutive androstane and pregnane x receptors.Toxicol Sci. 2014 Jun;139(2):501-11. doi: 10.1093/toxsci/kfu038. Epub 2014 Apr 1. Toxicol Sci. 2014. PMID: 24690595
-
Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator.Crit Rev Toxicol. 2014 Jan;44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Crit Rev Toxicol. 2014. PMID: 24180433 Free PMC article. Review.
-
Distinct Roles of the Sister Nuclear Receptors PXR and CAR in Liver Cancer Development.Drug Metab Dispos. 2022 Jul;50(7):1019-1026. doi: 10.1124/dmd.121.000481. Epub 2022 Feb 19. Drug Metab Dispos. 2022. PMID: 35184041 Review.
-
Mechanistic Insights of Phenobarbital-Mediated Activation of Human but Not Mouse Pregnane X Receptor.Mol Pharmacol. 2019 Sep;96(3):345-354. doi: 10.1124/mol.119.116616. Epub 2019 Jul 10. Mol Pharmacol. 2019. PMID: 31436536 Free PMC article.
Cited by
-
Epigenetic Mechanisms Contribute to Intraindividual Variations of Drug Metabolism Mediated by Cytochrome P450 Enzymes.Drug Metab Dispos. 2023 Jun;51(6):672-684. doi: 10.1124/dmd.122.001007. Epub 2023 Mar 27. Drug Metab Dispos. 2023. PMID: 36973001 Free PMC article. Review.
-
Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars.Biomolecules. 2024 Jul 15;14(7):850. doi: 10.3390/biom14070850. Biomolecules. 2024. PMID: 39062564 Free PMC article.
References
-
- Ariyoshi N, Imaoka S, Nakayama K, Takahashi Y, Fujita K, Funae Y, Kamataki T (2001) Comparison of the levels of enzymes involved in drug metabolism between transgenic or gene-knockout and the parental mice. Toxicol Pathol 29 (Suppl):161–172. - PubMed
-
- Aubrecht J, Hirsch-Ernst KI, Becker-Rabbenstein V, Kahl GF, Taniguchi H, Höhne MW (1995) Induction of cytochrome P-4502B1-related mouse cytochrome P-450 and regulation of its expression by epidermal growth factor/transforming growth factor α in primary hepatocyte culture. Biochem Pharmacol 50:781–785. - PubMed
-
- Bauer D, Wolfram N, Kahl GF, Hirsch-Ernst KI (2004) Transcriptional regulation of CYP2B1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol 65:172–180. - PubMed
-
- Bautista-Olivier CD, Elizondo G (2022) PXR as the tipping point between innate immune response, microbial infections, and drug metabolism. Biochem Pharmacol 202:115147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

