An allosteric modulator activates BK channels by perturbing coupling between Ca2+ binding and pore opening
- PMID: 36351900
- PMCID: PMC9646747
- DOI: 10.1038/s41467-022-34359-6
An allosteric modulator activates BK channels by perturbing coupling between Ca2+ binding and pore opening
Abstract
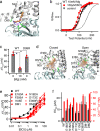
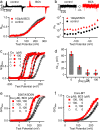
BK type Ca2+-activated K+ channels activate in response to both voltage and Ca2+. The membrane-spanning voltage sensor domain (VSD) activation and Ca2+ binding to the cytosolic tail domain (CTD) open the pore across the membrane, but the mechanisms that couple VSD activation and Ca2+ binding to pore opening are not clear. Here we show that a compound, BC5, identified from in silico screening, interacts with the CTD-VSD interface and specifically modulates the Ca2+ dependent activation mechanism. BC5 activates the channel in the absence of Ca2+ binding but Ca2+ binding inhibits BC5 effects. Thus, BC5 perturbs a pathway that couples Ca2+ binding to pore opening to allosterically affect both, which is further supported by atomistic simulations and mutagenesis. The results suggest that the CTD-VSD interaction makes a major contribution to the mechanism of Ca2+ dependent activation and is an important site for allosteric agonists to modulate BK channel activation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982;296:746–749. - PubMed
-
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. - PubMed
-
- Davies AG, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. - PubMed
-
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

