Achieving of high-diet-fiber barley via managing fructan hydrolysis
- PMID: 36351972
- PMCID: PMC9646770
- DOI: 10.1038/s41598-022-21955-1
Achieving of high-diet-fiber barley via managing fructan hydrolysis
Abstract
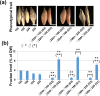
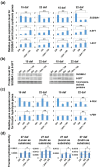
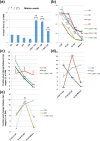
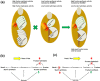
High fructan content in the grain of cereals is an important trait in agriculture such as environmental resilience and dietary fiber food production. To understand the mechanism in determining final grain fructan content and achieve high fructan cereal, a cross breeding strategy based on fructan synthesis and hydrolysis activities was set up and have achieved barley lines with 11.8% storage fructan in the harvested grain. Our study discovered that high activity of fructan hydrolysis at later grain developmental stage leads to the low fructan content in mature seeds, simultaneously increasing fructan synthesis at early stage and decreasing fructan hydrolysis at later stage through crossing breeding is an efficient way to elevate grain diet-fiber content. A good correlation between fructan and beta glucans was also discovered with obvious interest. Field trials showed that the achieved high fructan barley produced over seven folds higher fructan content than control barley and pull carbon-flux to fructan through decreasing fructan hydrolysis without disruption starch synthesis will probably not bring yield deficiency.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
High fructan barley lines produced by selective breeding may alter β-glucan and amylopectin molecular structure.Carbohydr Polym. 2023 Sep 15;316:121030. doi: 10.1016/j.carbpol.2023.121030. Epub 2023 May 16. Carbohydr Polym. 2023. PMID: 37321727
-
Fructan extracts from wheat stem and barley grain stimulate large bowel fermentation in rats.Nutr Res. 2012 Aug;32(8):599-606. doi: 10.1016/j.nutres.2012.06.009. Epub 2012 Jul 31. Nutr Res. 2012. PMID: 22935343
-
Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet.Molecules. 2024 Jan 5;29(2):282. doi: 10.3390/molecules29020282. Molecules. 2024. PMID: 38257195 Free PMC article.
-
Fructan biosynthesis in transgenic plants.J Exp Bot. 2003 Jan;54(382):549-67. doi: 10.1093/jxb/erg056. J Exp Bot. 2003. PMID: 12508066 Review.
-
Major cereal grain fibers and psyllium in relation to cardiovascular health.Nutrients. 2013 Apr 29;5(5):1471-87. doi: 10.3390/nu5051471. Nutrients. 2013. PMID: 23628720 Free PMC article. Review.
References
-
- Wagner W, Keller F, Wiemken A. Fructan metabolism in cereals: Induction in leaves and compartmentation in protoplasts and vacuoles. Z. Pflanzenphysiol. 1983;112:359–372. doi: 10.1016/S0044-328X(83)80053-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

