Anti-neoplastic sulfonamides alter the metabolic homeostasis and disrupt the suppressor activity of regulatory T cells
- PMID: 36352020
- PMCID: PMC9646802
- DOI: 10.1038/s41598-022-23601-2
Anti-neoplastic sulfonamides alter the metabolic homeostasis and disrupt the suppressor activity of regulatory T cells
Abstract
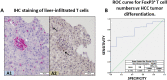
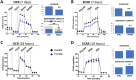
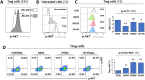
Regulatory T cells (Tregs) are essential to maintain self-tolerance and immune homeostasis but, as components of the tumor microenvironment (TME), are also a major barrier to effective cancer immunosurveillance and immunotherapy. FH535 and its derivative Y3 are two N-aryl-benzene-sulfonamides (NABs) that inhibit HCC cell proliferation and tumor progression. However, the impact of NABs on the immune cells in the TME is not yet known. Analyses of explanted livers from patients with hepatocellular carcinoma (HCC) showed that high levels of tumor-infiltrating Tregs were associated with poor tumor differentiation. These results lead us to investigate the immunomodulatory effects of NABs in regulatory and effector T cells. Exposure of primary human Tregs to NABs induced a rapid but temporary increase of cell expansion, a gradual disruption of suppressor activity, and concomitant bioenergetics and autophagic flux dysregulations. In contrast to Tregs, no gross effects were observed in effector T cells. Addition of Rapamycin prevented the functional decay of Tregs and restored their metabolic profile, suggesting that NAB effects require the integrity of the mTOR pathway. This study revealed the immunomodulatory properties of NABs with a preferential impact on Treg activity and provided novel insights into the anti-tumor potential of sulfonamides.
© 2022. The Author(s).
Conflict of interest statement
RG, DSW, CL and FM are holding patents IR-2321 and IR-2349 for NAB derivative Y3. Competing interest is not applicable for remaining authors.
Figures



References
-
- Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

