PANTHER: AZD8931, inhibitor of EGFR, ERBB2 and ERBB3 signalling, combined with FOLFIRI: a Phase I/II study to determine the importance of schedule and activity in colorectal cancer
- PMID: 36352028
- PMCID: PMC9902557
- DOI: 10.1038/s41416-022-02015-x
PANTHER: AZD8931, inhibitor of EGFR, ERBB2 and ERBB3 signalling, combined with FOLFIRI: a Phase I/II study to determine the importance of schedule and activity in colorectal cancer
Abstract
Background: Epidermal growth factor receptor (EGFR) is a therapeutic target to which HER2/HER3 activation may contribute resistance. This Phase I/II study examined the toxicity and efficacy of high-dose pulsed AZD8931, an EGFR/HER2/HER3 inhibitor, combined with chemotherapy, in metastatic colorectal cancer (CRC).
Methods: Treatment-naive patients received 4-day pulses of AZD8931 with irinotecan/5-FU (FOLFIRI) in a Phase I/II single-arm trial. Primary endpoint for Phase I was dose limiting toxicity (DLT); for Phase II best overall response. Samples were analysed for pharmacokinetics, EGFR dimers in circulating exosomes and Comet assay quantitating DNA damage.
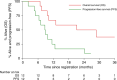
Results: Eighteen patients received FOLFIRI and AZD8931. At 160 mg bd, 1 patient experienced G3 DLT; 160 mg bd was used for cohort expansion. No grade 5 adverse events (AE) reported. Seven (39%) and 1 (6%) patients experienced grade 3 and grade 4 AEs, respectively. Of 12 patients receiving 160 mg bd, best overall response rate was 25%, median PFS and OS were 8.7 and 21.2 months, respectively. A reduction in circulating HER2/3 dimer in the two responding patients after 12 weeks treatment was observed.
Conclusions: The combination of pulsed high-dose AZD8931 with FOLFIRI has acceptable toxicity. Further studies of TKI sequencing may establish a role for pulsed use of such agents rather than continuous exposure.
Trial registration number: ClinicalTrials.gov number: NCT01862003.
© 2022. The Author(s).
Conflict of interest statement
TTN is Chief Medical Officer of Nano Clinical Ltd. PRB has shares in, and is a consultant for, Nano Clinical Ltd. All other authors declare no conflicts of interest.
Figures

References
-
- Scartozzi M, Mandolesi A, Giampieri R, Bittoni A, Pierantoni C, Zaniboni A, et al. The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab. Oncologist. 2011;16:53–60. doi: 10.1634/theoncologist.2010-0119. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

